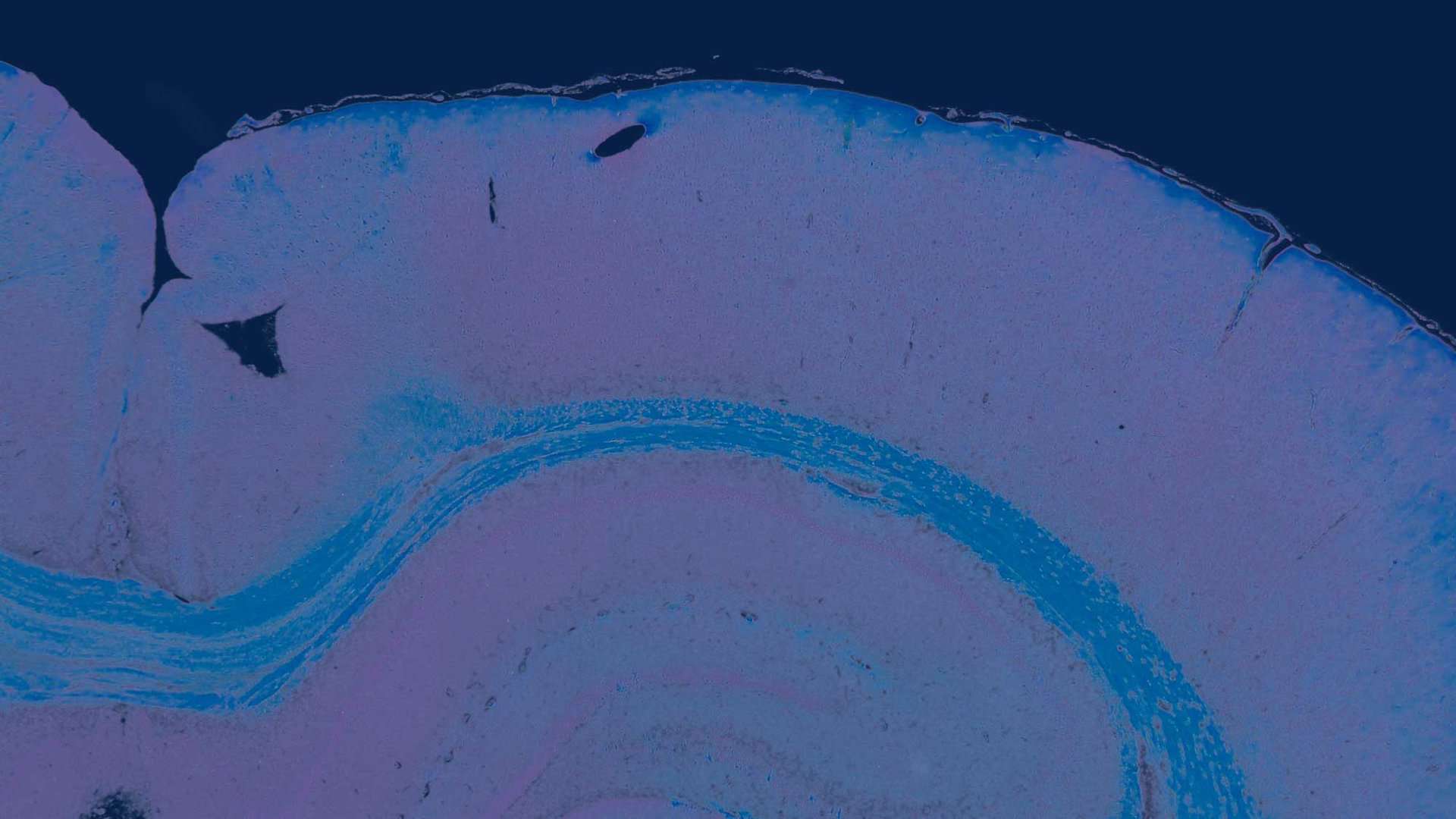
Pre-clinical (digital) pathology evaluation of drugs, vaccines and chemicals in toxicology studies for safety assessment
Global Pathology Support B.V.
Toxicologic Pathology
Vision statement
Drugs, chemicals and vaccines are safe and have no or least possible negative side effects.
Mission statement
At GPS B.V. we establish the safest dose of drugs, chemicals and vaccines by conducting toxicologic pathology research.
Proud member of:
NVVP – Dutch Society for Pathology
NVT – Toxicologic Society of the Netherlands
STP – Society of Toxicologic Pathology
SOT – Society of Toxicology
ESTP – European Society of Toxicologic Pathology

- 30+
Years of Experience
- 2000+
Clients Served
Global Pathology Support B.V.
Global Pathology Support (GPS) is a consultancy company, founded in 2004 in the Netherlands. GPS specializes in the pre-clinical pathology evaluation of chemicals in toxicology studies for safety assessment.
Our services focus on safety of products that are developed by the (bio-)pharmaceutical and chemical industry for registration of e.g. medicines, vaccines. GPS also provides services according to the Registration, Evaluation and Authorization of Chemicals (REACH) agreement for the safety evaluation of chemicals in compliance to the OECD-Guidelines.
As part of GPS continuing commitment to enable our clients to reach their development goals, we are now excited, next to conventional histopathology using the light microscope, to also deliver innovative GLP validated Digital Pathology; Full Histopathology Evaluations and Peer Review solutions from preclinical toxicology studies on a Global scale. This option (Digital Pathology) can now also be performed from scans of histology slides from a large number of whole slide image formats (e.g. Hamamatsu, 3DHistech, Olympus, Aperio/Leica, Ventana/Roche, Philips, Zeiss scanners etc).
Our Services
We offer you our services applying highest quality standards, in a flexible, fast and efficient way using one of the best Pathology Systems (Provantis® – Instem LSS) for histopathology assessment.
Digital Pathology
Also referred as Telepathology: Histopathology assessment by the toxicologic pathologist on Whole Slide Images (W.S.I.) from tissue glass slides.
Toxicologic Pathology consulting
Global Pathology Support is certified with the Endorsement of GLP-Compliance of the OECD principle of Good Laboratory Practice (including Digital Pathology). Histopathology assessment performed by a Board Certified Toxicologic Pathologist.
Biopharmaceutical consulting
Global Pathology Support has all the desired experience and prestige to conduct biopharmaceutical consulting.
Gross pathology assessment
Global Pathology Support is one of the few contract laboratories in The Netherlands, to be able to properly executing gross pathology assessment.
Neuropathology assessment
Global Pathology Support is well equipped with expert knowledge and experience in evaluating neuropathology of preclinical toxicity studies (e.g. OECD 443).
Peer review of Pathology
Throughout the years our service has expanded to histopathology reviews, Peer Review of Pathology (GLP), advice and consulting in toxicologic pathology and neuropathology evaluations.
Greetings, I am Dr. Bob Thoolen, a seasoned Board Certified Toxicologic Pathologist with over 25 years of expertise. At Global Pathology Support B.V. (GPS), we are at the forefront of ensuring the preclinical safety of medicines, vaccines, and chemicals. Our commitment lies in delivering high-quality, cost-competitive toxicologic pathology services in adherence to international guidelines such as OECD Good Laboratory Practice.
Connect with us at our headquarters in The Hague, Netherlands, or contact us to explore the depth of our pathology services. Join us in shaping the future of preclinical safety at Global Pathology Support B.V.
Dr. R.J.M.M. Thoolen
Board Certified Toxicologic Pathologist (PhD, CRP/TP) / Test Facility Manager
This is to state that I know Dr Thoolen for last several years as a colleague and friend. I have worked with him during his days as a principal pathologist at Solvay Pharmaceutical, BV, The Netherlands and later working at Global Pathology Support in The Netherlands. Robert is a well known toxicological pathologist with considerable experience in routine pathology work and peer review of reports. His dedication and quality of work is of high standards. Robert’s friendly approachable manners make him an easy person to work with.
He has worked well within the profession and his contributions to STP’ nomenclature group on the important topic of liver is well appreciated
I wish him success in his continued ventures in toxicological pathology.
Me and my colleagues always appreciated the collaboration with Bob as one of our consulting external pathologists and his reliable, fully GLP-compliant histopathology support by keeping thoroughly deadlines in a timely flexible manner.
We have repeatedly worked with Dr. Thoolen. He has always delivered accurate slide evaluations within a very compatible time frame. His polite and knowledgeable character made it easy for us to work with him, even in challenging projects.
Key-role of the Toxicologic Pathologist in Safety Evaluation at Global Pathology Support
From necropsy to histopathological evaluation, interpretation and use of digital pathology
Toxicological pathology – what does it mean and what is the role of the pathologist?
As regards the use of medicinal products from the pharmaceutical industry, the user’s experience should be positive and the negative side effects should be kept as minimal as possible. To evaluate negative effects, toxicological studies are carried out. Studies on the toxicity of substances aim to prevent diseases by setting limiting values, below which the health risk is negligible or nil. The (toxicological) pathologist plays a crucial role in assessing the safety of vaccines, medicines and chemicals. The emphasis is on the identification of pathological changes, the evaluation and relevance of these changes, and in particular the interpretation of these data from animal experiments in the context of their application to humans (risk assessment). Catastrophes in the last century, for example after using thalidomide (teratogenic effects) and the synthetic estrogen diethylstilbestrol (DES) in utero (carcinogen) have taught us how important it is to be alert to the side effects of drugs. Contamination of food sources by pesticides and industrial waste must also be monitored. Examples of compounds that have been shown to be carcinogenic in laboratory animals (usually in relatively high doses) and that end up in low amounts in human daily food are certain pesticides (DDT and dieldrin). Also industrially contaminated substances need thorough preclinical safety evaluations (e.g., Polychlorinated Biphenyls, Per- and Polyfluoroalkyl substances (PFAS). Moreover, the fact that more and more drugs are withdrawn from circulation after launch also highlights the importance of safety evaluation, in which the Toxicological Pathologist plays a crucial role.
At Global Pathology Support B.V. we provide preclinical pathology support in a GLP-compliant manner (Good Laboratory Practice) for the histopathological assessment of toxicology studies which plays a major role in the safety evaluation of compounds tested. We also use “Whole Slide Images“(W.S.I.’s) in the evaluation of non-clinical GLP-studies by the Board Certified Toxicologic Pathologist.
Whole slide images (W.S.I.’s) have become an essential modern tool for toxicologic pathology in the evaluation of non-clinical GLP-studies. The use of W.S.I.’s in toxicologic pathology has revolutionized the way pathological data is collected, analyzed, and communicated. The additional option for evaluating scans of slides (W.S.I.’s) by the Toxicologic Pathologist is more efficient and has other benefits.
Good Laboratory Practice (GLP)
Global Pathology Support (GPS) plays a crucial role in the delivery of sound scientific, accurate and GLP-compliant pathology data from preclinical toxicity and carcinogenicity studies. In order to do that, a GLP-compliant quality system is legally mandatory. GPS works according to Good Laboratory Practice for over 19 years now.
This guarantees our clients Good Laboratory Practice of pathology reports derived from GPS for over 38 countries world-wide. These reports can readily be used for registration according to the respective National Authorities.










