Authors Bob Thoolen Robert R.Maronpot Takanori Harada Abraham Nyska Colin Rousseaux Thomas Nolte David E.Malarkey Wolfgang Kaufmann Karin Küttler Ulrich Deschl Dai Nakae Richard Gregson Michael P.Vinlove Amy E.Brix Bhanu Singh Fiorella Belpoggi Jerrold M.Ward
Abstract
The INHAND Project (International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice) is a joint initiative of the Societies of Toxicologic Pathology from Europe (ESTP), Great Britain (BSTP), Japan (JSTP) and North America (STP) to develop an internationally-accepted nomenclature for proliferative and non-proliferative lesions in laboratory animals. The purpose of this publication is to provide a standardized nomenclature and differential diagnosis for classifying microscopic lesions observed in the hepatobiliary system of laboratory rats and mice, with color microphotographs illustrating examples of some lesions. The standardized nomenclature presented in this document is also available for society members electronically on the internet (http://goreni.org). Sources of material included histopathology databases from government, academia, and industrial laboratories throughout the world. Content includes spontaneous and aging lesions as well as lesions induced by exposure to test materials. A widely accepted and utilized international harmonization of nomenclature for lesions of the hepatobiliary system in laboratory animals will decrease confusion among regulatory and scientific research organizations in different countries and provide a common language to increase and enrich international exchanges of information among toxicologists and pathologists.
Keywords
Diagnostic pathology; hepatobiliary system; histopathology; liver; nomenclature; rodent pathology.
Abbreviations
AE1/AE3, Two clones of anti-cytokeratin monoclonal antibodies; a.k.a., Also known as; AS, Anterior Segment; α-SMA, α -smooth muscle actin; Bcl-2, B-cell lymphoma 2 – apoptosis regulator protein; BSTP, British Society of Toxicological Pathologists; CD (31, 34, 68), Cluster differentiation (31, 34, 68); CEA, Carcinoembryonic antigen; CK, Cytokeratin; ED1, Rat homologue of human CD68; EM, Electron microscopy; ESTP, European Society of Toxicologic Pathology; Factor VIII, Blood clotting factor/anti-hemophilic factor; F4/80, Rat anti-mouse macrophage monoclonal antibody; H&E, Hematoxylin and Eosin; IHC, Immunohistochemistry; JSTP, The Japanese Society of Toxicologic Pathology; Ki-67, Nuclear protein associated with proliferation; LAMP, Lysosome associated protein; LLL, Left lateral lobe; LML, Left medial lobe; MIB-1, Monoclonal antibody that detects K-67 antigen on formalin fixed paraffin embedded sections; MS, Middle Segment; NTP, National Toxicology Program; NLDC-145, Rat anti-mouse dendritic cell monoclonal antibody; NOS, Not otherwise specified; OX-6, MHC Class II Ia antibody; PAS, Periodic acid-Schiff; PC, Caudate Process; PCNA, Proliferator Cell Nuclear Antigen; PCR, Polymerase Chain Reaction; PP, Papillary Process; PPA, Processus papillaris anterior; PPAR, Peroxisome Proliferator-Activated Receptor; PS, Posterior Segment; RER, Rough Endoplasmic Reticulum; RLL, Right lateral lobe; RML, Right medial lobe; SRA-E5, Mouse monoclonal anti-macrophage antibody for Scavenger Receptor A; SOPs, Standard Operating Procedures; STP, Society of Toxicologic Pathology.

LLL = Left lateral lobe; RLL = Right lateral lobe; PC = Caudate Process; LML = Left medial lobe; RML = Right medial lobe; PP = Papillary Process; PPA = Processus papillaris anterior; AS = Anterior Segment; MS = Middle Segment; PS = Posterior Segment.
Gray, Williams, and Bannister (1995); Browning, Schroeder, and Berringer (1974); König, Sautet, and Liebich (2004); Rajtová, Horák, and Popesko (2002); Vons et al., 2009.
a (Number of lobes / Number of lobes including segments).
I. General Introduction
The liver is a major target organ in safety assessment of preclinical toxicity and oncogenicity studies with rodents; hence, hepatic pathology is central to many toxicological pathology studies. As toxicologic pathologists sometimes experience difficulties in distinguishing the wide variety of liver lesions in the rodents for safety evaluation purposes, this document is a consensus of senior toxicologic pathologists regarding suggested nomenclature that should be used for specific lesions.
Standardized diagnostic criteria and nomenclature are essential to harmonize the classification and reporting of hepatic non-proliferative as well as proliferative lesions. This INHAND document serves as a framework that can be used for the harmonization of diagnostic criteria of hepatic lesions in laboratory rats and mice. These recommendations for diagnostic criteria and preferred terminology should not be considered mandatory; proper diagnoses are ultimately based on the discretion of the toxicologic study pathologist.
The INHAND (International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice) initiative creates a framework for the harmonization of diagnostic nomenclature (classification of lesions using the same terminology) in different rodent organ systems. It is a joint initiative between Societies from the United States (STP), Great Britain (BSTP), Japan (JSTP), and European countries (ESTP).
This document is organized to provide introductory material that reviews comparative interspecies differences in anatomy and liver function, followed by a listing of liver lesions in a standardized format. The liver lesions descriptions include differential diagnoses to aid in distinguishing primary diagnoses from similar appearing lesions. Throughout the document, comparisons are made with respect to similar liver lesions that may occur in humans. It should be noted that the preferred diagnostic terminology for some lesions in this document might represent departures from traditional nomenclature schemes found in standard textbooks. Furthermore, illustrative photomicrographs for a given diagnostic entity may occasionally depict additional tissue changes as this reflects actual situations frequently observed in pathological evaluation of toxicity studies.
II. Anatomy
The liver occupies the cranial third of the abdominal cavity and is comprised of multiple lobes; however, the nomenclature for the liver lobes varies among authors. There are basically left, middle, right, and caudate lobes (Harada et al. 1999; Eustis et al. 1990). A thin connective tissue capsule that is externally lined by peritoneal mesothelial cells covers the parietal and visceral surfaces of the liver. The middle lobe has an incomplete fissure where the falciform ligament attaches. In mice the gallbladder is located in the middle lobe fissure, whereas the rat does not have a gallbladder. The right lobe has an anterior and posterior component and the small caudate lobe consists of two or more disc like sublobes (See Figure 1).
Nomenclature for liver lobes varies among species and sometimes among authors. A table showing differences in liver lobes between species is included based on current anatomic features (Table 1).
III. Histomorphology
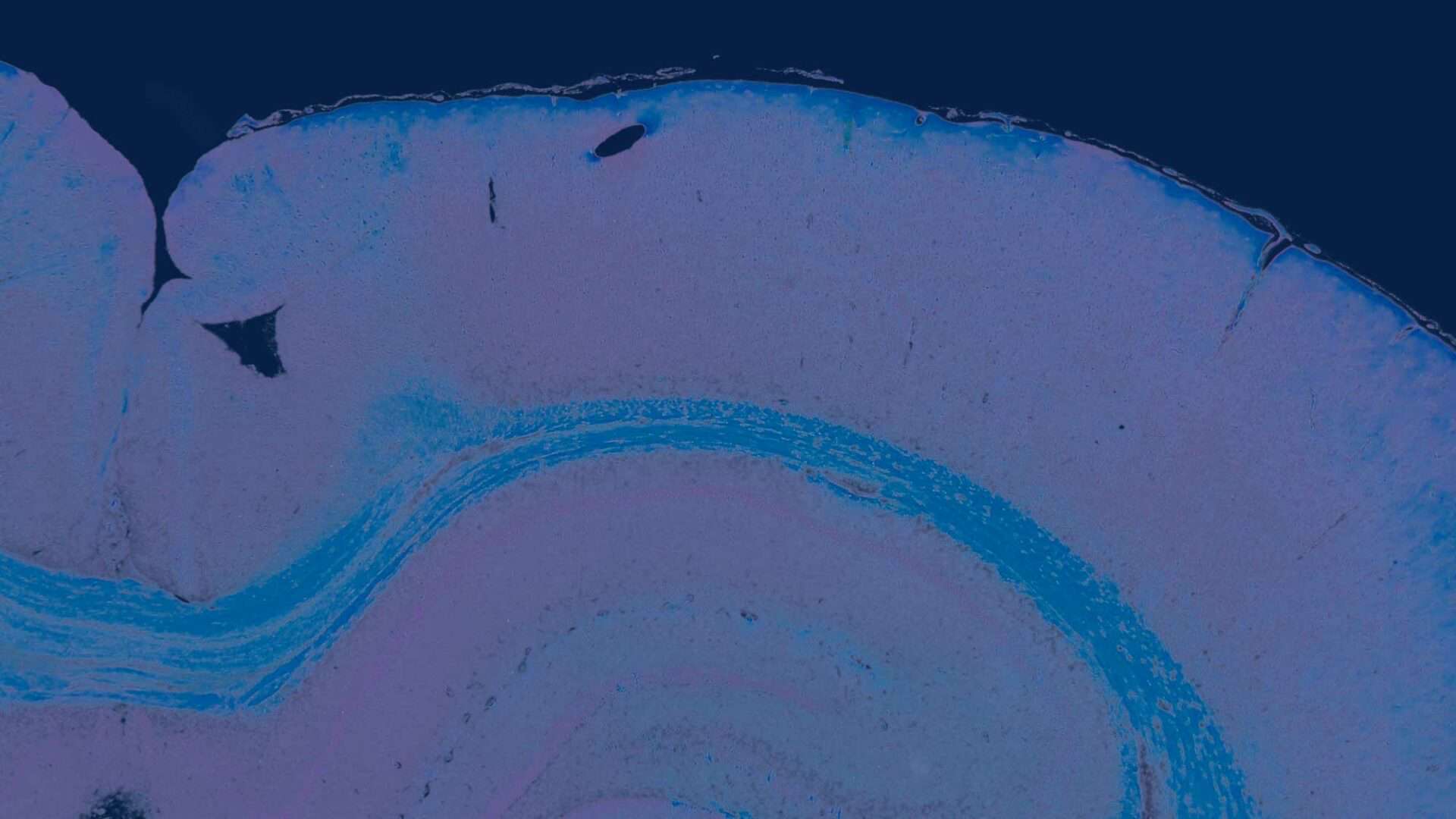
The two-dimensional microarchitecture of the liver has been categorized in at least three perspectives (Figure 2). The anatomic model is the classical lobule, a hexagonal structure divided into concentric centrilobular, midzonal, and periportal segments. The triangular portal lobule is based on bile flow and is centered on the portal triad (portal canal). The elliptical or diamond shaped liver acinus is a functional sub unit of the liver. It incorporates blood flow and metabolic functions and is divided in zone 1 (periportal), zone 2 (transitional; midzonal), and zone 3 (centrilobular). Functionally, zone 1 hepatocytes are specialized for oxidative liver functions such as gluconeogenesis, β-oxidation of fatty acids, and cholesterol synthesis, while zone 3 cells are more important for glycolysis, lipogenesis, and cytochrome P-450–based drug detoxification.
1. Blood Supply and Bile Flow
The liver has a dual blood supply, the hepatic portal vein and the hepatic artery. The hepatic artery supplies oxygenated blood. Approximately 75% of the blood is delivered to the liver via the hepatic portal vein that drains the spleen, stomach, intestines, and pancreas. Branches of the hepatic artery and portal vein are seen in the portal triads along with bile ducts and are separated from the hepatic cords by a ‘‘limiting plate’’ of hepatocytes. The bile ducts join to form the hepatic duct leading to the small intestine in rats and to the gallbladder in mice. Blood flows from the portal areas to the central vein in the center of each lobule while bile flows from the center of the hepatic lobule to the portal areas and on to the hepatic duct.
2. Histology
The two most commonly used descriptions for the structural and functional units of the liver are the hepatic lobule (Kiernan 1883) and the acinus (Rappaport et al., 1954) (Figure 2). The structural unit, the hepatic lobule, is modeled on the blood flow within the liver and is commonly used for descriptive pathology and morphological diagnoses. The functional unit, the hepatic acinus, is modeled on blood flow and metabolism within the liver. More recently a parenchymal unit in the liver has been described as a cone-shaped three-dimensional structure comprised of approximately fourteen hepatic lobules supplied and drained by common vascular tributaries (Malarkey et al. 2005; Teutsch, Schuerfeld, and Groezinger 1999; Teutsch 2005). This parenchymal unit more closely explains the random size and shape distribution of the more classical hepatic lobule as seen in a conventional two-dimensional histology slide. It also provides a basis for understanding the heterogeneous response of various hepatic lobules to chemical insult.
In addition to hepatocytes, the liver is comprised of a variety of cell types, including biliary cells, endothelial cells, Kupffer cells, Ito cells (stellate cells), fat-storing cells, and pit cells in addition to hematopoietic cells in the sinusoids and blood vessels. Polyhedral hepatocytes comprise approximately 60% of the liver arranged in plates or cords that radiate from the central vein to the portal areas. In two-dimensional sections they are typically one cell layer thick and form anastomoses (Miyai 1991). On one surface they are separated from the sinusoidal wall by a peri-sinusoidal space, the space of Disse, where they are exposed to tissue fluids. On the opposite side of the hepatocyte bile canaliculi are formed with hepatocytes in an adjacent hepatic cord. Desmosomes, gap junctions, and stud-like protrusions connect contiguous hepatocytes within a cord. Biliary cells form bile ducts in the portal areas and constitute the portal triad with a hepatic artery and a portal vein. Fenestrated endothelial cells line the sinusoids and synthesize prostaglandins. Kupffer cells are a self-renewing fixed macrophage comprising approximately 10% of all liver cells (Eustis et al. 1990). Kupffer cells are phagocytic, secrete mediators of inflammation, and catabolize lipids and proteins. Ito cells (stellate cells) are peri-sinusoidal cells that store vitamin A and are also a major source of collagen in the liver. Pit cells are lymphocytes that have natural killer activity and are primarily located in periportal areas (Wright and Stacey 1991).
3. Immunohistochemistry
Immunohistochemistry (IHC), utilizing fluorescent or chromogen tagged antibodies, is a useful adjunct for identification of different cell types in the liver. Selected examples are provided in Table 2.
Use of IHC can be helpful for diagnostic purposes and is common in human pathology where panels of immunohistochemical stains are used for supporting diagnoses. Not all commercially available preparations of a given antibody will react the same way between different laboratories and between different species. Furthermore, expertise is required for tissue handling to unmask cellular antigens that may be cross-linked during tissue fixation. Diagnostic evaluation of immunostains typically requires inclusion of both positive and negative controls. The interpretations of IHC results are usually performed in conjunction with histopathological findings and sometimes also with consideration of gross findings and/or clinical pathology or other relevant study results.
IV. Physiology
The liver is responsible for maintenance of many homeostatic and physiological functions. Liver size is governed both by genetic factors and by the rate of biochemical activity to maintain optimal functional mass. It is an organ system capable of rapid responses to a variety of noxious stimuli. Following loss of hepatocytes from stimuli such as transient toxic insult, infection, or partial hepatectomy, the liver is rapidly restored to its optimal mass to maintain normal function.
Liver functions are complex and diverse including endocrine and exocrine activity, metabolism, conjugation, detoxification, and hematopoiesis in early embryonic and fetal development (Harada et al. 1999). The liver is continuously exposed to all ingested substances absorbed through the intestinal tract via the portal vein and systemically via the arterial blood supply. A pivotal hepatic function in toxicologic pathology is xenobiotic biotransformation that leads to detoxification of materials absorbed in the intestinal tract. Xenobiotic metabolism by hepatocytes can occur by phase I (often the cytochrome oxidase series) and phase II reactions (often the formation of the water-soluble glucuronide) (Graham and Lake 2008; Martignoni, Groothuis, and de Kanter 2006). Hepatic metabolic processes may also cause indirect toxicity by generating electrophilic species capable of reacting with proteins, nucleic acids, and other cytoplasmic organelles (Xu, Li, and Kong 2005). Intrinsic and induced enzymes responsible for hepatic function may be unevenly distributed throughout the hepatic lobule and between the different lobes (Greaves 2007). The presence of background changes and undercurrent disease states affects the hepatic and biliary morphology, for example, caloric restriction diminishes hepatocellular size and can make interpretation of test-article-related changes more challenging. Other factors that influence the liver morphology are: body weight loss, blood flow, food intake, vascular and hemodynamic changes, timing and duration of exposure, withdrawal effects, and functional heterogeneity. Functional heterogeneity expresses itself via differences in metabolism, oxygen supply, β-oxidation, amino acid metabolism, gluconeogenesis, glycolysis, ureagenesis, liponeogenesis, and bile acid and bilirubin secretion. These factors can affect occurrence of non-proliferative as well as proliferative liver lesions in rodents.

Geller, Dahll, and Alsabeh (2008); Malhotra, Sakhuja, and Gondal (2004); Hurlimann and Gardiol (1991); Davenport et al. (2001); Kashiwagi, Kaidoh, and Inoué (2001); Faa et al. (1998).
V. Liver Necropsy and Trimming Protocol
At necropsy, rat and mouse liver may be weighed and individual liver lobes examined carefully for gross lesions. In conventional preclinical rodent studies, gross lesions must be correlated with the histopathological findings. Liver-specific trimming protocols (see Figure 1) according to standard operating procedures (SOPs) are used (e.g., see Ruehl-Fehlert et al. 2003). Dissected lobes and trimmed liver pieces can be fixed in 10% neutral buffered formalin (no more than 1 cm thick in 1:10 tissue: formalin).
VI. Grading of Liver Lesions
Interpretation of hepatic lesions in safety assessment studies requires consideration of gross and microscopic findings, hematology, clinical chemistry, and liver weights in the concurrent control groups of animals and should take into account species and strain, age, caging, diet, and tissue sampling.
Many pathologists use a grading system to document lesion severity. In toxicological pathology, the generation of ordinal data using a scoring system allows statistical analysis for effects and trends (Gad and Rousseaux 2002). However, not all grading systems are the same and may differ in how they incorporate distribution, stage, and extent of lesions. The problem of harmonization as it relates to lesion severity has been recognized and discussed in some detail (Hardisty and Eustis 1990; World Health Organization 1978).
Most toxicologic pathologists use a common grading scale such as marginal or minimal, slight, moderate, marked, and severe for inflammatory, necrotizing, or other degenerative and responsive lesions. Tissue-specific locators are often used, such as portal, periportal, midzonal, centrilobular, hilar, ductal, periductal, peri-canalicular, or subcapsular to indicate the lesion distribution within the liver. Focal, multifocal, and diffuse are commonly used modifiers in the morphological diagnosis for distribution parameters. Based on the formal definition, a focal lesion refers to one specific area, or focus, whereas multifocal refers to more than one focus (foci). However, some pathologists use focal for both focal and multifocal, referring to the nature of the lesion rather than its actual distribution and using grading to reflect the extent of the multifocality. Schemes for scoring lesion severity vary widely and no single system is likely to be accepted by all pathologists. While a sample grading scheme for focal and multifocal liver lesions is provided in Table 3, this should not be regarded as a universal or specific INHAND-recommended grading scheme.

liver lesions (modified from Hardisty and Eustis 1990; World
Health Organization 1978; Derelanko 2000).
VII. Nomenclature, Diagnostic Criteria, and Differential Diagnosis
A. Congenital Lesions
Introduction
Developmental anomalies occasionally occur in the liver of rodents. These malformations might be expressed in different forms and be of different origin. They mostly occur as isolated effects and are considered by the pathologist in distinguishing background hepatic lesions versus xenobiotic-induced lesions that occur in rodent preclinical toxicity studies.
Hepatodiaphragmatic Nodule (Figures 3 and 4)
Pathogenesis: Developmental alteration.
Diagnostic features:
- Visible grossly and tinctorially similar to normal hepatic parenchyma.
- Rounded extensions usually of the medial lobe(s).
- Increased mitoses, cytological alterations, and nuclear alterations may be present.
- Linear chromatin structures with small lateral projections are pathognostic.
Differential diagnosis:
- Hepatocellular focus of cellular alteration—tinctorial variation from normal parenchyma and does not protrude into the diaphragm.
- Hepatocellular neoplasia—when visible grossly does not protrude into the thoracic cavity.
- Regenerative hyperplastic nodule (nodular hyperplasia)—typically involves multiple nodules of hyperplasia separated by proliferative bands of oval cells or connective tissue.
Comment: Hepatodiaphragmatic nodules can be seen in rats at any age and their occurrence in fetuses is considered presumptive evidence of a congenital origin. While they appear to be protruding through the diaphragm and extending into the thoracic cavity, they actually are attached to and covered by a thin fibrous portion of the diaphragm (Eustis et al. 1990).
An incidence ranging from 1% to 11% has been reported for hepatodiaphragmatic nodules in Fischer 344 rats (Eustis et al. 1990), with few cases reported in other rat stocks and strains. Mice do not develop such nodules but may have focal lesions similar to those in rat hepatodiaphragmatic nodules and with large nuclei with large central nucleoli-like basophilic bodies.
B. Hepatocellular Responses, Cellular Degeneration, Injury, and Death
Introduction
The function and structure of most liver cells are relatively constrained by their genetic programs of metabolism, differentiation, and specialization. While the cells of the hepatic parenchyma have the flexibility to adapt to changing physiological demands with reversible functional and morphological alterations, sufficient stress, or noxious stimuli may lead to inability to maintain homeostasis and adverse cellular adaptations. The morphological response to injurious stimuli depends on the nature of the injury and its severity and duration. Often at high doses, targeted cells go through a sequence of cellular degeneration followed by cell death, but at lower doses degenerative changes do not necessarily lead to cell death. Consequentially, cellular changes that do not lead to cell death or death of the animal may be called ‘‘adaptive’’ changes that can be considered either adverse or not adverse reactions, depending on the nature of the change. There are cellular adaptations involving metabolic or functional alterations that lead to increases in cellular organelles and intracellular accumulations of a variety of endogenous and exogenous substances but allow the cell and animal to survive and often live normally. Similar changes may occur in human liver, such as cholestasis, a common lesion in human liver after long-term drug therapy. However, in animals, when the limits of adaptive responses are exceeded or do not occur in response to chemical exposure, irreversible cellular injury and cellular death occurs, with possible subsequent illness and death.
Adaptive changes or doses of chemicals that induce adaptive changes usually do not result in illness or death of rodents. Often these processes are dose and chemical related.
Fatty Change
Synonyms/subtypes: Lipidosis, vacuolation, lipid, macrovesicular and/or microvesicular steatosis, phospholipidosis.1
___________________________________
1 Electron microscopy or special staining needed for a definitive diagnosis.
Pathogenesis: Perturbations in lipid metabolism and disposition.
Diagnostic features:
Macrovesicular fatty change (Figures 5 and 6).
- Hepatocytes contain a large well-defined single rounded vacuole within each cell.
- Nucleus and cytoplasm displaced to the periphery.
- A few hepatocytes may contain one or more smaller vacuoles.
Microvesicular fatty change (Figure 7).
- Hepatocytes partially or completely filled with numerous small lipid vacuoles.
- Affected hepatocytes may have a ‘‘foamy’’ appearance.
- Small vacuoles do not normally displace the nucleus to the periphery in contrast to macrovesicular steatosis.
Differential diagnosis:
- Hydropic degeneration—clear cytoplasm without nuclear displacement.
- Glycogen accumulation—irregular and poorly defined lacy clear spaces in the cytoplasm (rarefaction) usually with centrally located nuclei.
Comment: There is a difference in preferred nomenclature among pathologists for this change. Based strictly on an H&E-stained section, a diagnosis of cytoplasmic vacuolation of hepatocytes is a universally acceptable descriptive diagnosis. Based on the experience of the observer, the specific morphological features of the cytoplasmic vacuolation may be sufficiently consistent with intracytoplasmic lipid accumulation to warrant a presumptive diagnosis of fatty change. The unequivocal demonstration of intracytoplasmic fat, however, requires a special stain.
Fatty change can be induced by a number of different agents and is usually divided into two main types, namely, microvesicular and macrovesicular, although mixed forms can frequently be observed (Greaves 2007; Gopinath, Prentice, and Lewis 1987; Goodman and Ishak 2006; Kanel and Korula 2005). Macrovesicular lipidosis is a reaction to a wide variety of injuries and can also be regarded as a physiological adaptation demonstrated as an imbalance between uptake of lipids from blood and secretion of lipoproteins by the hepatocyte (Goodman and Ishak 2006). Microvesicular lipidosis is usually indicative of more serious hepatic dysfunction but can also result from nutritional disturbances (Greaves 2007).
Specific xenobiotics can induce either macrovesicular or microvesicular lipidosis in humans (Kanel and Korula 2005). In animal studies, it is common to see a mixture of macrovesicular and microvesicular lipidosis. In those situations, one can either diagnose the most prevalent form or record the findings as mixed. Commentary in the pathology narrative report might be appropriate, especially if recording the most prevalent form of lipidosis. Liver with admixed presence of glycogen and fatty change can be observed (Figures 8 and 9).
Fatty change and necrosis may appear together although they may differ in proportion. A number of causes other than xenobiotic exposure, such as chronic hepatic injury, diet, metabolic and hormonal status, debilitation of animals, and fasting before necropsy, should be taken into consideration in reviewing these changes (Vollmar et al. 1999; Katoh and Sugimoto 1982; Nagano et al. 2007; Denda et al. 2002). The distribution can be either diffuse (e.g., ethionine) or zonal (e.g., centrilobular in CCl4; periportal in phosphorus toxicity; midzonal in choline deficiency). Inadequate fixation procedures may sometimes give rise to artifacts with microvesicular vacuolation, although mostly with less clear cytoplasm (Li et al. 2003).
Focal fatty change can sometimes be seen spontaneously and is usually described as such. A specific variation occurs near the attachment of the falciform ligament and gallbladder in mice and is referred to as ‘‘tension lipidosis’’ (Harada et al. 1999) (Figures 10 and 11). Spontaneous fatty change can differ between strains and is a normal finding in BALB mice. Livers of these mice are typically paler than in other strains. Focal fatty change in the liver of rodents has previously been categorized as vacuolated altered hepatic foci (Eustis et al. 1990), but current practice is to diagnose this change as focal fatty change rather than as a focus of hepatic alteration (Figures 12 and 13).
Fatty change can also be observed in combination with other hepatotoxic injuries (e.g., chronic liver toxicity, degeneration, inflammation, and necrosis) or nutritional disturbance (e.g., diet, vitamin A excess) in both animals and man. Special stains on cryostat sections can demonstrate fat (e.g., Oil red O or Sudan Black) (Jones 2002).
Phospholipidosis2
Synonym: Cytoplasmic vacuolation, foam cells.
___________________________________
2 Electron microscopy or special staining needed for a definitive diagnosis.
Pathogenesis: Induced by xenobiotics with a cationic amphophilic structure.
Diagnostic features:
- Multiple irregulars to round clear membrane-bound vacuoles.
- Tends to be a diffuse change affecting hepatocytes.
Differential diagnosis:
- Fatty change—round clear vacuoles tend to be single or multiple and discrete.
- Glycogen accumulation—irregular and poorly defined clear spaces in the cytoplasm (rarefaction) usually with centrally located nuclei; positive stained with periodic acid-Schiff staining.
Comment: Definitive diagnosis of phospholipidosis is not possible based strictly on H&E-stained liver sections. A diagnosis of cytoplasmic vacuolation of hepatocytes will typically be an acceptable descriptive diagnosis. Since the cytoplasmic vacuolation may mimic microvesicular fatty change, a descriptive diagnosis of cytoplasmic vacuolation is recommended in the absence of electron microscopy or special immunostaining.
Phospholipidosis can be induced by xenobiotics with a cationic amphophilic structure (Halliwell 1997; Anderson and Borlak 2006; Reasor, Hastings, and Ulrich 2006; Chatman et al. 2009) (Figures 14 and 15). It is a lipid storage disorder seen when complexes between xenobiotics and phospholipids accumulate within lysosomes. Phospholipidosis refers to a specific form of hepatic vacuolation with the occurrence of concentric membrane bound lysosomal myeloid bodies/lamellar bodies that can be confirmed by specific staining and electron microscopy (Hruban, Slesers, and Hopkins 1972; Obert et al. 2007) (Figure 16). Definitive diagnosis requires electron microscopy or positive immunostaining. Immunohistochemical staining for a lysosomal-associated protein and adipophilin may be used to differentiate phospholipidosis from conventional fatty change (Obert et al. 2007). Both preexisting neutral fat and phospholipids can be observed in combination. The macrovesicular and the microvesicular fatty change (vacuolation) generally located at the cell periphery stains positively for Oil Red-O and the membranes surrounding these lipid vacuoles stain positively for adipophilin (a protein that forms the membrane around non-lysosomal lipid droplets) but negative for LAMP-2 (a lysosome-associated protein) by immunohistochemical techniques (Obert et al. 2007). This indicates that this vacuolation was due to accumulation of nonlysosomal neutral lipid. Cytoplasmic microvesiculation located centrally in hepatocytes that exhibit positive immunohistochemical staining for LAMP-2 (Figure 17) but is negative for Oil-Red-O and adipophilin is indicative of phospholipid accumulation (Obert et al. 2007).
Amyloidosis (Figures 18 and 19)
Pathogenesis: Cellular process related to misfolding of protein.
Diagnostic features:
- Deposition of pale, homogeneous, amorphous eosinophilic material.
- Deposition often peri-sinusoidal, periportal, or involving blood vessel walls.
- Localization is extracellular.
Comment: This is a rare condition in rats but is a more common age-related phenomenon in hamsters and mice (Greaves 2007; BSTP 2007). The basis of the pathological change is the cell’s inability to prevent protein misfolding, to revert misfolded proteins to normal, or to eliminate misfolded proteins by degradation. This can result in deposition of potentially cytotoxic protein aggregates of amyloid as in other protein aggregation diseases (Aigelsreiter et al. 2007). The amyloid is predominantly composed of protein in a beta-pleated sheet conformation.
The incidence of spontaneous amyloidosis usually increases with age and is common in CD-1 mice (Harada et al. 1996). Amyloid observed in the liver often is referred to as secondary amyloidosis (serum amyloid A protein) and is seen in the sinusoids and within the portal vessel walls. Hepatocytes adjacent to sinusoidal amyloid deposits are often atrophic. A number of factors (e.g., species, age, strain, gender, endocrine status, diet, stress, and parasitism) can influence the occurrence of amyloidosis (Beregi et al. 1987; Coe and Ross 1990; Lipman et al. 1993; Harada et al. 1996; Liu et al. 2007). Other organs are often involved in the deposition of amyloid (e.g., kidney, nasal submucosa, lamina propria intestines, heart, salivary gland, thyroid, adrenal cortex, lung, tongue, testis, ovary, and aorta).
Amyloidosis can be confirmed with additional histochemical staining (Congo red) where it shows pink-red staining and apple green birefringence under polarized light (Vowles and Francis 2002; Kanel and Korula 2005) and by immunohistochemistry.
Mineralization (Figure 20)
Pathogenesis: Hypercalcemia secondary to diet or abnormal calcium metabolism; hepatocellular necrosis (dystrophic mineralization).
Diagnostic features:
- Intra- or extracellular basophilic deposits, sometimes with calcification.
Differential diagnosis:
- Artifact—hematoxylin stain deposits in clear spaces.
- Pigment deposits—may be tinctorially different from mineralization and often seen within macrophages.
- Intrabiliary accumulation of test compound or metabolite.
- May be associated with necrosis, inflammation, or neoplasia.
Comment: Mineralization is rarely seen in the liver and gallbladder in rodents. Dietary factors (mineral content) and disturbance of calcium metabolism commonly influence the process of hepatic mineralization (Harada et al. 1999; Spencer et al. 1997; Yasui, Yase, and Ota 1991; DePass et al. 1986). Mineralization can sometimes be observed in combination with inflammation or neoplasia (Harada et al. 1999; Kanel and Karuda 2005). Mineral deposits can be demonstrated by using additional stains (Alizarin Red, von Kossa) (Churukian 2002).
Pigmentation (Pigment Deposition) (Figures 21–25)
Pathogenesis: Incidental occurrence and secondary to cellular and erythryoid breakdown products; lipid peroxidation of cellular membranes; altered heme metabolism.
Diagnostic features:
Lipofuscin:
- Pigment can be seen in hepatocytes as well as in Kupffer cells.
- May vary from pale yellow to deep granular brown.
- May be sudanophilic with autofluorescence under ultraviolet light.
- Often located adjacent to bile canaliculi.
Iron/hemosiderin:
- Can be yellow to brown.
- May be finely granular.
- Usually appears intracellularly in Kupffer cells and hepatocytes.
Porphyrin:
- Pigment is dense dark brown to red-brown and when viewed with polarization is bright red with a centrally located dark ‘‘Maltese cross.’’
- Brilliant red fluorescence when viewed in fresh frozen sections; fades with exposure to ultraviolet light.
- Most often located in bile ductules and bile canaliculi.
Bile (cholestasis) (Figures 23–25):
- Appears as elongated pale green-brown plugs within bile caniculi.
- Will appear in Kupffer cells following rupture of caniculi.
- Can appear as finely granular pigment within in hepatocytes, which is common in human liver but much less common in rodents.
- Not a common xenobiotic response in rodents; more common in humans and monkeys.
Differential diagnosis:
- Artifact—hematoxylin stain deposits in clear spaces.
- Formalin precipitated pigment—extracellular granular yellow-brown deposits often associated with erythrocytes.
- Test compound/metabolite—may be distinctive for the specific compound.
- Mineralization—basophilic deposits; may be associated with calcification.
Comment: A number of different pigments may be seen as an incidental finding within hepatocytes and Kupffer cells in rodents. Some of them may increase and/or accumulate after treatment. Definitive diagnosis of a specific pigment typically requires special stains.
Lipofuscin or ceroid is sometimes referred to as ‘‘wear and tear’’ or ‘‘aging’’ pigment and therefore is often observed in older animals. It is considered to represent a breakdown of cell membranes. Lipofuscin accumulates in postmitotic and aging cells. It has been shown to be a mixture of oxidized proteins and lipids, carbohydrates, and trace amount of metals (Seehafer and Pearce 2006). A variety of stimuli can accelerate the accumulation of this pigment, such as drug and chemical exposure, trauma and circulatory factors, and diet (Greaves 2007). Lipofuscin accumulation in the liver may be augmented by certain chemicals (Kim and Kaminsky 1988; Marsman, 1995). Treatment of rats with PPAR alpha agonists such as fenofibrate and associated increased lipid peroxidation seen in rodents treated with hypolipidemic agents can induce lipofuscin accumulation in liver after prolonged treatment (Nishimura et al. 2007; Goel, Lalwani, and Reddy 1986; Reddy et al. 1982). Increased lipofuscin accumulation has also been observed in partially hepatectomized liver of rats (Sigal et al. 1999). Lipofuscin is insoluble in alcohols and xylene and other solvents normally used in the preparation of slides. Special stains such as Smorl’s can be used to demonstrate the pigment. Storage granules appear gray with Sudan Black B, may be PAS-positive, and may stain with Luxol fast blue and Ziehl-Neelsen (Jones 2002).
Porphyrin pigment, a precursor of heme protein, is seen with treatment of some xenobiotics. Bile pigment is a common finding when there is cholestasis secondary to obstruction of bile flow or when there is perturbation in bile metabolism. Bile pigment stains green with Hall’s method.
Hemosiderin pigment represents precipitated iron that is most frequently generated as a breakdown product of erythrocytes and is derived from hemoglobin and accumulates in the liver following local or systemic excess of iron. Deposition or iron may occur following excess dietary intake or treatment by xenobiotics (Popp and Cattley 1991; Greaves 2007; Travlos et al. 1996). Excess of iron following injection may be stored as hemosiderin and deposited in the reticuloendothelial component of the liver (and other organs such as spleen and bone marrow) (Bruguera 1999; Pitt et al. 1979). Intraperitoneal injection of aflatoxin B1 can also induce hemosiderosis in hamsters (Ungar, Sullman, and Zuckerman 1976). Endogenous iron deposition can be found following breakdown of blood cells (hemolytic event). Iron pigment can be found in Kupffer cells, macrophages, and hepatocytes. In hepatocytes, the iron is stored in the form of ferritin (ferric iron bound to protein apoferritin) (Popp and Cattley 1991). A spontaneous inherited predisposition for hepatic iron pigmentation has been reported in Sprague-Dawley rats (Masson and Roome 1997), and iron deposition can be found in the aging mouse liver (Harada et al. 1996). Iron can be demonstrated using Perls’ Prussian blue stain in which iron stains blue.
Hemosiderin slowly dissolves in acids, especially oxalic acid. Non-aldehyde fixatives can remove hemosiderin or alter it in such a way that reactions for iron are (false) negative (Churukian 2002). Malarial pigment is seen in hepatocytes and Kupffer cells of Plasmodium sp experimentally infected mice. It is the pigment from the organism and not hemosiderin.
Porphyrin pigment normally occurs in tissues only in small amounts and is a precursor of the heme portion of hemoglobin (Churukian 2002). Porphyrin deposition in the liver of rodents is found after administration of a number of compounds including griseofulvin where it can be seen in association with hepatocellular neoplasia (Stejskal et al. 1975; Zatloukal et al. 2000; Knasmuller et al. 1997; Tschudy 1962). Griseofulvin administration in mice may result in inhibition of the mitochondrial enzyme ferrochelatase and (compensatory) induction of ALA synthetase. Griseofulvin-induced accumulation of porphyrins in mouse liver is followed by cell damage and necrotic and inflammatory processes (Knasmuller et al. 1997). Proto-porphyrin pigment in liver of rats and mice is mainly found in the bile ducts and leads to bile duct proliferation and portal inflammation, but can also occur in hepatocytes, Kupffer cells, and portal macrophages (Hurst and Paget 1963). The birefringence of porphyrin appears to be associated with bilamellar components within the pigment (Stejskal et al. 1975). This pigment is also seen in combination with liver fibrosis and cirrhosis, bile duct proliferation, periportal inflammation, and hepatocarcinogenesis (Kanel and Korula 2005; Hurst and Paget 1963; Greaves 2007; Rank, Straka, and Bloomer 1990).
Crystals (Figures 26–28)
Pathogenesis: Hyperlipidemia (cholesterol crystals), Chi313 (Ym1) protein (eosinophilic biliary crystals).
Diagnostic features:
- Rhomboid or needle-like structures often birefringent under polarized light.
- Needle-like crystals in the mouse can be intracellular or extracellular and may be associated with intense eosinophilic epithelial cytoplasm and extracellular crystals of various sizes.
Differential diagnosis:
- Artifact—wispy blue hematoxylin deposits in clear spaces.
Comment: In hyperlipidemia, cholesterol crystals can deposit in the liver with or without granulomatous inflammation (Greaves 2007; Graewin et al. 2004; Handley, Chien, and Arbeeny 1983). During gall stone formation, in addition to classical rhomboid-shape monohydrate crystals, cholesterol can also crystallize transiently as needle-, spiral-, and tubule- shaped crystals of anhydrous cholesterol (Dowling 2000). Eosinophilic crystals have been described in intrahepatic bile ducts and gallbladder of different laboratory mice strains, and some of these crystals have been shown to contain chitinase-like proteins confirmed by immunohistochemistry for Ym1 protein (now Chi313) (Ward et al. 2001; Harbord et al. 2002).
Crystal formation may be associated with inflammatory and/or proliferative bile duct changes and fibrosis in mice and may also occur spontaneously (Lewis 1984; Rabstein, Peters, and Spahn 1973; Enomoto et al. 1974). Numerous crystals can be demonstrated using a simple system of polarizing microscopy. Crystals are capable of producing plane-polarized light, thus showing birefringence.
Inclusions, Intranuclear, and Cytoplasmic (Figures 29–32)
Synonyms: Inclusion bodies, intranuclear cytoplasmic invagination, acidophilic inclusions, globular bodies.
Pathogenesis: Protrusion of cytoplasm into an invagination of the hepatocyte nuclear membrane without the actual protrusion necessarily being present in the plane of section. Seen in specific viral infections. Deposition of protein material within hepatocyte cytoplasm.
Diagnostic features:
- Intranuclear inclusions are round, distinct, usually eccentrically located, and may partially or almost completely fill the nucleus.
- Contents of intranuclear inclusion bodies are often eosinophilic and may be granular or flocculent.
- Intracytoplasmic inclusions are round to oval, homogenous, eosinophilic, and occur as single or multiple structures in the cytoplasm.
Differential diagnosis:
- Enlarged nucleolus—one or more deeply basophilic structures in normal size nuclei.
- Viral inclusion bodies (cytomegalic virus, experimental viral infections).
- Cytoplasmic vacuole artifact—postmortem plasma influx (Li et al. 2003).
Comment: Both intranuclear and intracytoplasmic inclusions are common findings in the aging mouse liver and may be seen in normal as well as neoplastic hepatocytes (Percy and Barthold 2001; Frith and Ward 1988; Irisarri and Hollander 1994). When the intranuclear inclusions represent invaginations of the cytoplasm into the nucleus, they may contain cytoplasmic organelles in electron micrographs (van Zwieten and Hollander 1997). Ultrastructurally, three types of cytoplasmic inclusions have been described: dense reticulated substance in the dilated cisternae of rough endoplasmic reticulum, fine granular substance in rough endoplasmic reticulum, and non–membrane bound dense granulofibrillar in the cytoplasm (Helyer and Petrelli 1978).
Kakizoe, Goldfarb, and Pugh (1989) have correlated the incidences of cytoplasmic inclusion with hepatocellular tumors in different mice strains. C57BL/6 mice are relatively more resistant to hepatocarcinogens than C3H and C57BL/6 x C3H F1 mice. The tumors in the C57BL/6 mice were unique in their early focal development of cells containing inclusions. The authors suggested that the higher incidence of inclusions in liver might be related to slowing of the tumor growth leading to lower incidence of hepatocellular tumors in C57BL/6 mice. Other types of intracytoplasmic inclusions such as Mallory bodies, lamellated, and crystalloid inclusions have been described in mice treated with different chemicals and in lysosomal storage diseases (Gebbia et al. 1985; Meierhenry et al. 1983; Rijhsinghani et al. 1980; Shio et al. 1982).
Cytoplasmic vacuoles can occur in hepatocytes and endothelial cells in a postmortem time-dependent manner in fasted and non-fasted rats (Li et al. 2003). This artifact is especially common in rats that are not exsanguinated at necropsy and the cytoplasmic vacuoles represent plasma influx into to affected cells (Figure 33). This artifact is more common in males than in females.
Hypertrophy, Hepatocellular (Figures 34–41)
Synonyms: Hepatocytomegaly.
Pathogenesis: Metabolic enzyme induction causing increase in endoplasmic reticulum; increase in peroxisomes; increase in mitochondria.
Diagnostic features:
- Enlarged hepatocytes may be tinctorially distinct.
- Cytoplasm may be homogeneous or granular.
- Zonal pattern of distribution (centrilobular, periportal, midzonal) may be present.
- Involving most or all lobules.
- Loss of hepatocellular plate architecture is possible.
- Sinusoidal compression.
- Concurrent degeneration and/or single cell necrosis is possible.
Differential diagnosis:
- Hepatocellular neoplasia—expansile mass with altered growth pattern; loss of lobular structure.
- Regenerative hyperplastic nodules—altered growth pattern; distorted lobular pattern.
- Foci of cellular alteration—usually a discrete collection of cells within the hepatic parenchyma.
- Hepatocellular degeneration—affected cells have increased cytoplasmic granularity and eosinophilia.
- Hepatocellular storage disease.
- Polyploidy—enlarged nuclei and/or binuclear hepatocytes are often associated with increased cytoplasmic volume.
Comment: Hepatocellular hypertrophy, secondary to increase in microsomal enzymes often occurs with a zonal or specific lobular pattern and commonly occurs following exposure to enzyme inducing xenobiotics. There is enlargement of the hepatocyte cytoplasm secondary to increase in the cytosolic protein or number of organelles (e.g., smooth endoplasmic reticulum, peroxisomes, mitochondria). Classically hepatocyte hypertrophy occurs without increase in hepatocyte numbers or DNA (i.e., hyperplasia or polyploidy), however, combinations with increased mitoses do occur (e.g., PPAR-alpha agonists).
Hepatocellular hypertrophy following enzyme induction is considered an adaptive response to chemical stress. Strain differences in responsiveness occur. While typically an adaptive response, excessive hypertrophy from enzyme induction of hypertrophy can lead to hepatocellular degeneration and necrosis. Hepatocellular hypertrophy may be associated with increase in absolute liver weights. Enzyme induction leading to hepatocellular hypertrophy may be accompanied by some evidence of transient mitogenesis. Hypertrophy that is panlobular may be difficult to appreciate histologically because the contrast provided by a sublobular pattern is not evident. In some cases, hepatocyte hypertrophy related to metabolic enzyme induction may not be evident to the pathologist when liver weight increase is small for a group, for example, less than 20%.
Hepatocellular enlargement or swelling may occur from accumulation of glycogen, fat, or other substances and may also be a feature of degeneration and some forms of hepatocellular necrosis. To avoid confusion with the more common usage of hepatocyte hypertrophy for physiological enzyme induction, it is recommended that alternative forms of hepatocyte enlargement not be diagnosed as hepatocellular hypertrophy.
Hepatocellular Atrophy (Figures 42 and 43)
Pathogenesis: Inanition, starvation, hemodynamic changes, or pressure atrophy from neoplasia.
Diagnostic features:
- Decreased size of hepatocytes.
- Small liver trabeculae with decreased cytoplasmic volume, close proximity of hepatocyte nuclei, close proximity of portal tracts, and increased basophilia.
- Hepatocyte nuclei may be smaller than normal.
- May have a zonal distribution.
- May be associated with hepatocellular degeneration and/or single cell necrosis.
- May be associated with increased sinusoidal size.
- Depletion of cytoplasmic glycogen.
Differential diagnosis:
- Shrinkage artifact—retraction of softer tissue from firmer tissue.
- Artifact of fixation or processing—poorly stained tissue with loss of normal structure.
Comment: Hepatocellular atrophy can be caused by a number of factors such as inanition, starvation, hemodynamic changes, or pressure atrophy from neoplasia (Yu et al. 1994; Gruttadauria et al. 2007; Belloni et al. 1988). Hepatocyte atrophy may be associated with decrease in absolute liver weights in rats (Belloni et al. 1988). Ultrastructurally atrophic hepatocytes have reduced amounts of glycogen and decreased numbers of mitochondria.
Degeneration
Introduction: In the diagnostic lexicon, degeneration is a nonspecific diagnosis that provides limited useful information unless qualified to reflect the dominant morphological features. It is often at the borderline between adaptation with resolution back to normal structure and function and inability to adapt leading to cell death. In human clinical medicine degenerative disease most often refers to chronic debility involving organs and tissues that slowly accumulate damage over time. In rodent studies, degeneration may also be applicable to chronic debility, but more often it is used to reflect acute or chronic cytological alterations with characteristic morphological features. Combinations of different degenerative features may occur with or without inflammation and/or necrosis.
Based on H&E-stained sections, distinction between different forms of degeneration, hepatocellular hypertrophy secondary to enzyme induction, other forms of hepatocellular enlargement such as glycogen accumulation/retention, and even early necrosis (a.k.a. onconosis) may be difficult. In some cases, special stains may be required to more clearly delineate the nature of the cytologic alteration. Based strictly on H&E staining, a descriptive diagnosis of cytoplasmic alteration is recommended in lieu of interpretative diagnosis such as granular degeneration and hyaline degeneration. However, there are some degenerative lesions, such as hydropic degeneration and cystic degeneration, that are more clearly established in traditional pathology literature. The preferred diagnosis will be influenced by morphological features, conventional pathology practice, and the experience of the pathologist.
Glycogen accumulation in hepatocytes is a type of cytoplasmic alteration manifested on H&E-stained paraffin sections as clear spaces in the cytoplasm and a centrally located nucleus. Intracellular accumulation of glycogen is a normal physiological response following food ingestion. Since rodents eat primarily in the evening hours, the largest amount of glycogen will be present during early morning hours. Intrahepatocyte glycogen is mobilized throughout the day, initially being removed from centrilobular hepatocytes. Consequently, the amount present varies depending upon whether the animals were fasted and on the time of necropsy during the day. Failure to accumulate glycogen because of inanition or abnormal glycogen retention may result from treatment-induced metabolic perturbations.
Cytoplasmic Alteration (Figure 44)
Synonyms: Cytoplasmic alteration, cytoplasmic change, granular change, granular degeneration, hyaline degeneration, glycogen accumulation; ground glass change.
Pathogenesis: Often xenobiotic-induced and may be associated with other forms of liver damage.
Diagnostic features:
- Affected cells may show increased cytoplasmic granularity, cell swelling, and eosinophilia.
Differential diagnosis:
- Artifact of fixation or processing—poorly stained tissue with loss of normal structure.
- Hepatocyte hypertrophy—cytoplasmic volume increased with uniform finely granular texture; usually associated with microsomal enzyme induction or peroxisome proliferation.
- Cytoplasmic vacuole artifact—postmortem plasma influx.
- Coagulation necrosis—loss of cytoplasmic and nuclear detail.
Comment: What has been described as granular degeneration can be seen in combination with other forms of liver damage (e.g., necrosis, hydropic degeneration, inflammation) (Huang et al. 2007; Gokalp et al. 2003; Datta et al. 1998; Xu et al. 1992; Aydin et al. 2003). Hepatocellular granularity may be due to swelling of cell organelles or increase in the numbers of cell organelles including peroxisomes, mitochondria, and smooth endoplasmic reticulum. Some pathologists do not consider granular degeneration to be a distinct entity and do not include it in their diagnostic lexicon.
Hyaline degeneration has been described by a number of authors, sometimes in combination with Mallory body formation (Gonzalez-Quintela et al. 2000; NTP Toxicology and Carcinogenesis Studies Ethylene Glycol 1993; Peters et al. 1983; Bruni 1960; Shea 1958; Omar, Elmesallamy, and Eassa 2005; Lin et al. 1996), but is rarely used as a separate description since a combination of findings is often present. Cytoplasmic alteration reflecting plasma influx is an artifact seen in nonexsanguinated rats in a postmortem time-dependent manner (Li et al. 2003) (see Figure 33).
Degeneration, Hydropic (Figure 45)
Synonyms: Cytoplasmic alteration, cytoplasmic change, hydropic change, cloudy swelling.
Pathogenesis: Intracytoplasmic fluid accumulation secondary to disturbance of cell membrane integrity.
Diagnostic features:
- Cytoplasmic vacuolation and ‘‘ballooning’’ with a centrally located nucleus.
- Lobular location may be centrilobular or periportal with increased clear cell change and cell swelling.
Differential diagnosis:
- Cytoplasmic vacuole artifact—postmortem plasma influx.
- Glycogen accumulation—hepatocytes not markedly enlarged; cytoplasmic clear areas are irregular.
Comment: Because of disturbance of the cell membrane integrity, accumulation of intracytoplasmic fluid may occur. This causes vacuolation and ‘‘ballooning’’ of cells. This change can be caused by a number of xenobiotics with differing lobular localization and may be a precursor to hepatocyte necrosis (Gkretsi et al. 2007; Wang et al. 2007; Peichoto et al. 2006; Matsumoto et al. 2006; Chengelis 1988).
Degeneration, Cystic (Figure 46 and 47)
Synonyms: Spongiosis hepatis (traditional diagnostic term preferred by many pathologists).
Pathogenesis: Cystic enlargement of perisinusoidal stellate cells (Ito cells) particularly observed in aging rats.
Diagnostic features:
- Multi-loculated cyst(s) lined by fine septa containing fine flocculent eosinophilic material (PAS-positive).
- The cysts are not lined by endothelial cells and do not compress the surrounding liver parenchyma.
- May be accompanied by occasional erythrocytes or leukocytes.
- May be observed within altered hepatic foci and liver tumors.
- Affected cells may be markedly enlarged.
Differential diagnosis:
- Angiectasis (Peliosis hepatis; vascular ectasia)—dilated endothelial lined vascular spaces that often contain blood cells.
- Sinusoidal dilatation—sinusoidal structure evident and spaces lined by endothelial cells.
- Necrosis—loss of nuclear and cytoplasmic detail and loss of stain affinity.
- Hemangioma—expansile structure lined by flattened endothelial cells; may be associated with parenchymal compression.
- Hemangiosarcoma—expansile mass lined by plump endothelial cells and/or layers of endothelial cells and associated with destruction of hepatic parenchyma.
Comment: Spontaneous and xenobiotic-induced cystic degeneration/spongiosis hepatis may occur in rats (Karbe and Kerlin 2002; Bannasch 2003; Babich et al. 2004; Newton et al. 2001). It is more common in aging rats with some male predilection. It is less common in mice. This lesion may be seen in or associated with other hepatic lesions (necrosis, regeneration; foci of cellular alteration; hepatocellular neoplasms). The pathogenesis is not fully understood (Bannasch, Block, and Zerban 1981; Karbe and Kerlin 2002).
Cell Death (Necrosis, Apoptosis)
Introduction: In the fully developed organism, cell death is the ultimate result of irreversible cellular injury. Cellular death in the liver is manifested by a spectrum of morphological patterns that can occur alone or in combinations. However, there are two primary manifestations of cell death: necrosis and apoptosis. For decades a form of necrosis involving individual isolated hepatocytes has been diagnosed as ‘‘single cell necrosis.’’ This particular change is now regarded as ‘‘apoptosis’’ by most pathologists (Levin 1999; Levin et al. 1999; Elmore 2007) when the majority of injured cells have the typical apoptotic morphology. Provided that there is no accompanying inflammatory reaction, the two terms are synonymous. However, since a diagnosis of ‘‘apoptosis’’ implies a specific sequence of biochemical and morphological events and should ideally be supported by electron microscopy, it may be more prudent to diagnose single cell necrosis unless there is definitive proof of apoptosis provided by electron microscopy (Levin et al. 1999). It can be mentioned in the pathology narrative that the observed ‘‘single cell necrosis’’ is morphologically consistent with ‘‘apoptosis.’’
Pathogenesis: Direct or indirect cellular damage, including anoxia. Apoptosis (a.k.a., single cell necrosis) can occur spontaneously in liver and may also be exacerbated or induced by treatment.
Single Cell Necrosis (Apoptosis) (Figures 48–50)
Diagnostic features:
- Affected hepatocytes may have condensed hyper eosinophilic cytoplasm and a somewhat angular outline.
- Not associated with an inflammatory response unless there is simultaneous necrosis.
- May occur spontaneously with one or two affected hepatocytes present in an occasional hepatic lobule.
- May be exacerbated by treatment.
- In standard H&E-stained sections, apoptotic hepatocytes (apoptotic bodies) are usually rounded with condensed cytoplasm.
- Rounded apoptotic bodies are typically surrounded by a clear halo.
- Fragments of nuclear material may be present within affected cells.
- Apoptotic bodies are frequently phagocytosized by adjacent normal cells including hepatocytes and macrophages.
Differential diagnosis:
- Small foci of necrosis—typically cells are swollen and there is loss of membrane integrity; usually not rounded; less intensely stained than apoptotic bodies; may be accompanied by inflammatory cells.
Comment: Apoptosis is a form of genetically controlled ‘‘programmed cell death.’’ Microscopically in H&E-stained tissue sections, apoptosis appears as dense eosinophilic shrunken cell bodies with maintenance of membrane integrity, nuclear fragmentation and cytoplasmic budding, and without an inflammatory response. Definitive diagnosis of apoptosis can be made by histological findings and confirmed by distinctive electron microscopic features. Consequently, use of a diagnosis of ‘‘single cell necrosis’’ is appropriate based strictly on H&E staining. The use of TUNEL kits or caspase immunostaining may assist in diagnosing apoptosis and enumerating affected cells, but necrosis may also be immunopositive. Inhibition of apoptosis also plays a key role in the process of carcinogenesis (Foster 2000). Although apoptosis can be observed spontaneously in the liver, certain chemicals may be able to trigger direct stimulation of pro-apoptotic pathways in hepatocytes (Feldmann 1997; Reed 1998). Apoptosis can also accompany treatment-related zonal necrosis in the liver, especially in situations where there may be a xenobiotic induced effect (Cullen 2005; Greaves et al. 2001).
While apoptosis represents a specific genetically programmed form of cell death unaccompanied by an inflammatory response, there are situations where small numbers of cells and even occasional single cells characterized by cell swelling can undergo necrosis without an inflammatory response. This represents an early stage of conventional necrosis, may occur within hours after exposure to a xenobiotic, and should not be diagnosed as single cell necrosis (apoptosis). A more appropriate diagnosis for this situation is focal necrosis (see the following).
Necrosis, Focal/Multifocal (Figures 51–53)
Diagnostic features:
- Single or multiple foci of a few pale staining hepatocytes.
- Usually retain normal morphological outline.
- May be associated with inflammation.
- May have an irregular distribution but can also occur in the subcapsular areas with minimal or no inflammation.
- Early lesions typically consist of three or four hepatocytes, but as the lesions progress more hepatocytes may be involved.
- Subcapsular necrosis may sometimes be observed in combination with hypertrophy.
Differential diagnosis:
- Foci of extramedullary hematopoiesis—mature and/or immature erythroid and myeloid cell aggregates without accompanying hepatocyte necrosis.
- Foci of inflammatory cell infiltrate—aggregates of cells, usually mononuclear cells, in absence of obvious hepatocellular necrosis.
- Infectious disease (MHV, Ectromelia, Clostridium pilliforme, Helicobacter hepaticus, Parvo virus, Noro virus)—a spectrum of acute to chronic active inflammation, degenerative, and proliferative changes specific for the infectious disease entity.
Comment: Some pathologists use focal for both focal and multifocal, referring to the nature of the lesion rather than its actual distribution. A severity grade can be used to reflect the multifocal nature of the lesion. Focal, multifocal, and subcapsular necrosis is occasionally seen in untreated rodents and may be a terminal event potentially due to hypoxic change secondary to impaired blood flow. Subcapsular necrosis has also been reported from direct pressure secondary to gastric distention and from some types of restraint (Parker and Gibson 1995; Nyska et al. 1992).
Necrosis, Zonal (Centrilobular, Midzonal, Periportal, Diffuse)
Pathogenesis: Secondary to direct or indirect damage from xenobiotic exposure; tissue anoxia.
Centrilobular (Figures 54–59)
Sometimes referred to as periacinar necrosis, it consists of irreversible cell death of centrilobular hepatocytes and is often seen after anoxia, or exposure to tannic acid, chloroform, or other hepatotoxic agents (Gopinath, Prentice, and Lewis 1987). This zone (Rappaport zone 3) is particularly vulnerable to ischemic damage because of its low oxygen gradient and generation of toxic metabolites due to high content of xenobiotic metabolizing enzymes (Comporti 1985; Walker, Racz, and McElligott 1985).
Diagnostic features:
- Early necrotic hepatocytes are swollen.
- Cytoplasm has increased eosinophilia.
- Nucleus undergoing lysis, not pyknosis.
- May have a minimal associated inflammatory reaction.
- Can be accompanied by glycogen depletion, hydropic degeneration, fatty change, hemorrhage, and ‘‘ballooning’’ of hepatocytes.
Midzonal (Figures 60–61)
This necrosis is the least common form of zonal necrosis and is mediated by specific toxicants (e.g., furan, concavalin-A, beryllium) (Wilson et al. 1992; Boyd 1981; Seawright 1972; Satoh et al. 1996; Cheng 1956). The location is considered specific and has a metabolic basis.
Diagnostic features:
- Seen as a band of swollen and eosinophilic cells intermediate to the central vein (zone III) and the portal triad (zone I).
- Nucleus undergoing lysis.
- Two to three cells in thickness in the middle of the lobule.
Periportal (Figure 62)
Hepatic necrosis in the periportal zone is observed following a variety of agents (e.g., phosphorus, ferrous sulphate, allyl alcohol) (Kanel and Korula 2005; Atzori and Congiu 1996; Sasse and Maly 1991). Affected cells may encircle the portal tract (Popp and Cattley 1991) and may be associated with inflammatory and other changes (Ward, Anver, et al. 1994; Ward, Fox, et al. 1994; NTP Technical Report on the Toxicology and Carcinogenesis Studies of a Binary Mixture 2006; NTP Technical Report on the Toxicology and Carcinogenesis Studies of 2, 3, 7, 8-tetracholorodibenzo-p-dioxin 2006).
Diagnostic features:
- Swollen and/or eosinophilic hepatocytes may completely encircle the portal tract.
- Nucleus undergoing lysis.
- May be accompanied by periportal inflammation, fibrosis, bile duct proliferation, and oval cell hyperplasia.
Diffuse (Figures 63 and 64)
Synonym: Massive necrosis, panlobular necrosis.
Diagnostic features:
- Necrosis involving a large portion of a liver lobe.
- May be associated with torsion of a liver lobe.
- May be randomly distributed throughout the liver without a specific lobular localization.
Differential diagnosis:
- Autolysis—loss of microscopic tissue structure and stain affinity; pale eosinophilic staining and absence of nuclear detail.
- Torsion of a liver lobe—Affects an entire liver lobe, loss of microscopic structure.
- Infarction—usually an angularly shaped wedge or area of tissue necrosis; may be associated with a nearby thrombus.
Comment: Zonal necrosis is typically associated with exposure to xenobiotics that either directly damage hepatocytes or cause damage following metabolic activation by endogenous or induced enzymes. There is often a concentration gradient within the hepatic lobule with more extensive lobular involvement associated with higher doses of the toxic agent.
Hepatocellular necrosis can occur spontaneously in rodents or be induced by xenobiotics, toxins, or following treatment at high dosages with associated tissue anoxia, circulatory derangements, and biliary stasis. Necrosis (centrilobular, midzonal, periportal) might be accompanied by other histological changes (fatty change, congestion, hemorrhage, inflammation, bile stasis, fibrosis, etc.) to form a myriad of pathological changes. The distribution might also cross certain zones and may manifest as ‘‘bridging necrosis’’ showing confluence of the lesions (e.g., central to central veins, portal tract to portal tract, or portal tract to central zones). Bridging necrosis may ultimately give rise to bridging fibrosis.
A specific form of necrosis, ‘‘piecemeal necrosis’’, is characterized by necrosis of the limiting plate of the portal tract at the interface of hepatocytes and connective tissue of the portal tract, usually accompanied by inflammation, can be immune-mediated, and is seen in mice with resemblance to chronic active hepatitis in man (Kitamura et al. 1992; Nonomura et al. 1991; Kuriki et al. 1983).
Karyocytomegaly and/or Multinucleated Hepatocytes (Figures 65 and 66)
Synonyms: Karyocytomegaly, multinucleated hepatocytes, binucleated hepatocytes, karyomegaly, nuclear hypertrophy, hepatocytomegaly, polyploidy, anisonucleosis, anisokaryosis.
Pathogenesis: Duplication of nuclear material in absence of cytokinesis. Variations in nuclear size and ploidy (karyomegaly and/or anisokaryosis) are common in aging rodents.
Diagnostic features:
- Hepatocytes with either two or more nuclei or with a single enlarged nucleus which may be tetraploid or octaploid.
- Polyploid hepatocytes are frequently larger than adjacent diploid hepatocytes.
- Anisokaryosis is randomly distributed in the hepatic lobule with more affected hepatocytes in the centrilobular region.
Differential diagnosis:
- Hepatocellular neoplasia—mass or expansile proliferation of hepatocytes with distortion or loss of lobular architecture.
- Hepatocellular hypertrophy (enzyme induction)—increase in cytoplasmic volume not typically associated with increased nuclear size or number.
Comment: Karyocytomegaly is a reflection of hepatocyte polyploidy that occurs when there is duplication of nuclear material in the absence of cytokinesis. The result is an increase in the number of diploid nuclei per hepatocyte or an increase in the ploidy level of a single hepatocyte nucleus. Polyploidy increases with age in some strains of mice as well as following some treatment regimens resulting in hepatocytomegaly as well as karyomegaly (Harada et al. 1996). Variations in cell size as well as in nuclei and polyploidy are also common in aging rats of different strains. Karyomegaly and anisokaryosis are normal incidental findings, especially in older mice (Percy and Barthold 2001). Increase in cell size (cytomegaly) may accompany the increase in hepatocyte ploidy. Anisokaryosis (inequality in size of nuclei) is more common and dramatic in mice than in rats.
The development of polyploidy and its pattern vary among strains. C3H and DBA mice more commonly have octaploid cells with two tetraploid nuclei in adult liver while NZB and the out-bred strain NMRI at the corresponding age show a higher proportion of diploid cells with strikingly low proportions of tetraploid cells. Polyploidy has been observed in the early life (three weeks) in Ercc1 null mice. This premature polyploidy in Ercc1-deficient liver is most likely caused by increased levels of p21 in response to accumulating DNA damage (Chipchase, O’Neill, and Melton 2003). Toxic injury caused by chemicals such as phenobarbitone (Martin et al. 2001) and partial hepatectomy also induce an increase in ploidy, usually associated with extensive but transient hepatocyte proliferation (Gerlyng et al. 1993).
Although anisonucleosis (polyploidy) is known to occur as an age-related phenomenon, the nuclear and cellular changes can also be induced by xenobiotics (Schoental and Magee 1959; Jones and Butler 1975; Singh et al. 2007; Nyska et al. 2002; Guzman and Solter 2002; Lalwani et al. 1997; Travlos et al. 1996; Kari et al. 1995; Herman et al. 2002). In addition, multinucleated cells (formed by cell fusion rather than division) can be formed in rats after administration of 2, 3, 7, 8-tetrachloro-dibenzo-p-dioxin (Gopinath, Prentice, and Lewis 1987; Jones and Butler 1975). Eosinophilic cytoplasmic inclusions may be seen in affected hepatocyte nuclei because of cell membrane invaginations.
Cysts, Biliary (Hepatic Cysts) (Figures 67–69)
Pathogenesis: More common in aging animals occurs as a dilation of biliary structures.
Diagnostic features:
- Range in size from small to very large.
- Single or multiple cysts.
- Macroscopically may contain clear to pale yellow fluid.
- May occur anywhere in the liver and may be unilocular or multilocular.
- Multilocular cysts are divided into variably sized compartments by partial or complete connective tissue septa.
- Cyst walls are characteristically lined by flattened to cuboidal epithelium.
- May be mild compression of adjacent hepatic parenchyma.
Differential diagnosis:
- Cystic degeneration—consists of markedly enlarged cells with finely flocculent pale eosinophilic cytoplasm.
- Angiectasis (Peliosis hepatis)—dilated vascular spaces lined by endothelial cells; may contain blood cells.
- Bile duct dilation—dilated bile ducts lined by cuboidal epithelium; not multiloculated.
- Parasitic cyst—may have thickened wall and contain parasite tissue.
- Cholangioma—may cause compression of adjacent parenchyma and spaces lined by more endothelial cells than in biliary cysts.
Comment: Biliary cysts are commonly seen in older rats (Burek 1978; Greaves 2007; Harada et al. 1999). Solitary cysts can be observed without major adjacent morphology changes of the surrounding tissue. However, depending on the location, adjacent parenchyma may contain pressure atrophy of the hepatic cords of the liver, fibrosis, hemosiderin deposition, proliferation of bile ducts, or periportal lymphocytic infiltration. Single cysts are often caused by cystic dilatation of the intrahepatic bile ducts (Sato et al. 2005). Multiple cysts are observed also in hepatic polycystic disease, where they occur alone or in combination with polycystic kidney disease (Masyuk et al. 2004, 2007). They are often referred to as simple or multiloculated biliary cysts (Goodman et al. 1994). Polycystic liver can be observed in the rat (Muff et al. 2006; Sato et al. 2006) and hamster (Percy and Barthold 2001), resembling Caroli’s disease in humans (Clemens et al. 1980; Numan et al. 1986; Serra, Recalde, and Martellotto 1987). The cysts seen in polycystic disease are multiple and seen diffusely throughout the liver and are of variable size but generally large compared to the smaller biliary cysts.
C. Inflammatory Cell Infiltrates and Hepatic Inflammation (Hepatitis)
Introduction
A variety of focal, multifocal, and more generalized infiltrations of inflammatory cells are frequently present in liver tissue. Changes range from acute inflammatory cell infiltrate(s) or occasional aggregates of lymphocytes/lymphohistiocytic cells/foci of mononuclear cells without associated alterations of adjacent hepatocytes, to large panlobular patches of distinct hepatocyte necrosis accompanied by polymorphonuclear and mononuclear (lymphocytes, plasma cells, macrophages) cellular infiltrates. ‘‘Mononuclear cell’’ can be used when there is a mixture of cell types (lymphocytes; less often macrophages and plasma cells) or the cell type is mononuclear but cannot be unequivocally identified in H&E stain. If a cell type predominates, then the infiltrate should be classified as lymphocytic, plasmacytic, or histiocytic. While etiological agents (e.g., bacteria, virus, parasite) may be present, in most safety assessment studies the causes of significant inflammation are either cryptic or are attributed to a specific treatment regimen. Inflammatory reactions in the liver may be accompanied by oval cell and fibroblast proliferation and the propensity for hepatocellular proliferative responses to replace lost parenchyma.
It is recommended that use of the diagnostic term ‘‘inflammation’’ for the liver should be used sparingly. Liver inflammation (hepatitis) is operationally defined as a constellation of changes that represent a severe and generalized liver reaction and would require multiple diagnostic terms to adequately characterize (Figure 70). This type of reaction is not typically encountered in conventional rodent toxicity studies.
Traditionally, hepatic inflammatory responses have been classified as acute, subacute, chronic, granulomatous, and so on. These terms are somewhat interpretative, lack precise definitions, vary depending upon study duration, usually do not consist of a singular cell type, and do not have exclusive pathognomic features. A more descriptive approach is recommended and can be qualified by lesion distribution or use of subclassification and discretionary qualifiers (Figure 71).
Infiltration, Inflammatory Cell
Pathogenesis: Infiltration of different inflammatory cells is typically a response to parenchymal cell death with causes ranging from infectious agents, exposure to toxicants, generation of toxic metabolites, and tissue anoxia.
Infiltration, Neutrophil (Figure 72)
Synonyms: Inflammation, acute; acute inflammatory cell infiltrate(s), focus/foci of acute inflammatory cells; aggregate(s) of acute inflammatory cells.
Diagnostic features:
- Predominantly neutrophilic (and in some specific types of experiments, eosinophilic) cells present as focal aggregates often associated with dying hepatocytes or at the periphery of large areas of hepatocyte necrosis. A few lymphocytes and occasional macrophages may be present.
- Infiltrating cells are usually focal or multifocal.
- Necrosis of scattered individual cells or small clusters of cells without associated infiltrating neutrophils may also be present in some areas.
- Can be associated with whole regions of contiguous affected lobules and confluent hepatic necrosis extending between adjacent lobules (bridging hepatic necrosis).
- Hemorrhage may be associated with larger lesions.
- Oval cell proliferation may be present.
Differential diagnosis:
- Foci of extramedullary hematopoiesis—mature and/or immature erythroid and myeloid cell aggregates without accompanying hepatocyte necrosis.
- Granulocytic leukemia—hepatic parenchyma infiltrated and replaced by a monomorphic population of neutrophils or neutrophil precursors.
- Chronic inflammation—inflammatory cells include primarily mononuclear cell (largely lymphocytes, macrophages) and may include fibrosis and oval cell hyperplasia.
Comment: While neutrophilic infiltration in the liver is primarily a response to liver cell injury and necrosis, a few lymphocytes or macrophages may also be present. In addition, foci of neutrophils without apparent hepatocyte necrosis may be present, especially in situations of a transient effect on the liver. In florid reactions, areas of necrosis include degenerating neutrophils admixed with the necrotic hepatic parenchymal cells.
The extent of parenchymal cell death eliciting inflammatory cell infiltration varies from minimal microfocal lesions to large patches of coagulation necrosis encompassing multiple contiguous lobules. For xenobiotic-induced cell death and inflammation, the severity of the lesions is often a function of the dose of the hepatotoxicant. Apoptotic cell death may occur along with conventional necrosis. Depending upon etiology, acute inflammation can have a specific lobular distribution with the possibility of portal or centilobular bridging between adjacent lobules.
Infiltration, Mononuclear (Figures 73–79)
Synonyms: Inflammation, chronic; mononuclear cell aggregates; inflammation, granulomatous, focus/foci of mononuclear cells.
Pathogenesis: Persistent noxious stimuli associated with infection, toxic xenobiotics, continued low level parenchymal cell death, and immune mediated effects.
Specific subtypes may include: infiltration, lymphocytes; infiltration, histiocytes (monocytes); infiltration, plasma cells.
Diagnostic features:
- Infiltrating cells include lymphocytes, plasma cells, macrophages, and occasionally multinucleated giant cells.
- Infiltrating cells may be focal, multifocal, or diffuse in distribution.
- Randomly distributed aggregates of mononuclear cells (primarily lymphocytes) without any accompanying hepatocyte degeneration or necrosis may be common in older animals.
- Periportal aggregates of predominantly lymphoid cells in portal areas may be present.
- Evidence of liver cell necrosis may be minimal to mild with degeneration of scattered cells.
- Mononuclear cells with disruption of the limiting plate often infiltrate portal tracts.
- Bile duct hyperplasia may be present in some portal areas.
- An increase in mononuclear cells within sinusoids may be seen.
- Some capsular fibrosis and periportal fibrosis may be present.
- There may be evidence of hepatocellular regeneration.
Differential diagnosis:
- Histiocytic sarcoma—hepatic parenchyma infiltrated and replaced by collections of histiocytic cells that can be pleomorphic.
- Lymphoma—hepatic parenchyma infiltrated and replaced by a monomorphic population of lymphoid cells.
- Early lymphoma may mimic age-associated focal aggregates of lymphoid cells.
- Foci of extramedullary hematopoiesis—mature and/or immature erythroid and myeloid cell aggregates without accompanying hepatocyte necrosis.
- Viral hepatitis—may be difficult to distinguish from chronic (active) inflammation; evidence of a viral etiology can be supportive.
Comment: Mononuclear cell infiltration spans a wide spectrum of morphological features and severities depending upon the extent and duration of liver damage and any ongoing regenerative response. In distinction from acute inflammation, there are typically fewer neutrophils seen in chronic lesions and there is more involvement of portal areas with infiltrating mononuclear cells and disruption of limiting plates. Granulomatous inflammation with characteristic focal or multifocal nodular collections of mononuclear epitheloid cells and occasional giant cells associated with areas of hepatocyte necrosis can be considered a specific subtype of mononuclear cells infiltration and part of the spectrum comprising chronic inflammation of the liver (Figures 74–77). Some pathologists consider granulomatous inflammation of the liver to be a form of chronic inflammation when there is a prolonged duration with predominant presence of epitheloid cells/macrophages and prefer to address the fact that there may be areas containing mononuclear epitheloid cells and multinucleated giant cells in the chronic inflammatory process in an accompanying narrative. Fatty material and cholesterol deposits may accumulate in some granulomatous inflammatory reactions. Such localized accumulations of abundant cholesterol have been referred to as cholesterol granulomas (Figures 78 and 79).
When only few isolated collections of mononuclear cells are present, some pathologists may diagnose them as focal mononuclear cell aggregates. These focal accumulations are considered by some to be a background lesion, and for these aggregates using the cell type in the diagnosis, instead of inflammation or inflammatory cell infiltrate, may be preferable and less misleading. The frequency of these mononuclear cell aggregates may be exacerbated by treatment. Helicobacter sp. and murine norovirus infections in mice may cause these incidental lesions.
Infiltration, Mixed (Figure 80)
Synonym: Infiltration, purulent, and mononuclear; chronic active inflammation; mixed inflammatory cell focus/foci.
Diagnostic features:
- Infiltration of mononuclear cells along with neutrophils.
- Areas of active hepatocellular degeneration and/or necrosis often present.
- Evidence of hepatocellular regeneration may be present.
- Intralobular distribution is often random.
- May have diagnostic features common for both neutrophilic and mononuclear infiltration (see previous).
Differential diagnosis:
- Histiocytic sarcoma—hepatic parenchyma infiltrated and replaced by collections of histiocytic cells that can be pleomorphic.
- Lymphoma—hepatic parenchyma infiltrated and replaced by a monomorphic population of lymphoid cells.
Comment: Some pathologists consider a combined neutrophilic and mononuclear inflammatory cell infiltration as chronic active inflammation. This type of response is suggestive that the adverse stimulus is still present and/or that the immune system is active. Others may consider chronic active inflammation simply as a form of chronic inflammation with areas of neutrophilic infiltration in the inflammatory process and prefer to address that in their accompanying pathology narrative. A combined neutrophilic and mononuclear infiltrate (chronic active inflammation) is a common response found in mice chronically infected with Helicobacter hepaticus.
Infiltration, Peribiliary (Intrahepatic) (Figure 81)
Pathogenesis: Age associated change that may be exacerbated by treatment.
Diagnostic features:
- Peribiliary aggregates of inflammatory cells affecting a few to many portal areas.
- Sometimes accompanied by fibrosis.
- May be associated with some degree of bile duct hyperplasia.
Differential diagnosis:
- Cholangiofibrosis—proliferative and metaplastic biliary response plus fibrosis extending into the hepatic parenchyma; may be subcapsular.
Comment: A minimal to moderate peribiliary inflammatory cell infiltration consisting primarily of mononuclear cells can occur commonly in the livers of rats and mice and increases in incidence with animal age. Persistent obstruction to biliary flow may also lead to bile duct inflammation in hepatic portal areas. Although this background lesion may be considered a subtype of mononuclear cell infiltration (see previous), it is frequently diagnosed separately when exacerbated by treatment.
Fibrosis (Figures 82 and 83)
Pathogenesis: A reaction to acute or prolonged hepatotoxicity.
Diagnostic features:
- The presence of connective tissue in the liver above the normal low rate seen in portal areas.
- Peribiliary fibrosis particularly in aged rats.
- Three patterns of fibrosis are seen in the rodent liver: pericellular, peribiliary, and postnecrotic. The pericellular pattern is most common in mice.
- Normal hepatic architecture maintained.
- Increased prominence of fibrosis, which may surround hepatic lobules and bridge between adjacent portal areas.
- Hyperplastic nodules of hepatocytes separated by septae of connective tissue can be seen in rats but is less common in mice.
- Oval cell proliferation often spreading from periportal areas.
- Masson trichrome, van Gieson, or Silver stains can be used to delineate fibrosis.
Differential diagnosis:
- Cholangiofibrosis—marked pericholangial fibrosis with proliferation of biliary epithelium and metaplastic changes of glandular epithelium (e.g., goblet cells, Paneth cells).
- Regenerative hyperplasia—nodular growth pattern; distorted lobular pattern.
- Multiple foci of cellular alteration—discrete collections of cells within the hepatic parenchyma.
- Multiple hepatocellular adenomas.
- Scirrhous carcinoma—neoplastic epithelial cells will be embedded in a collagenous connective tissue proliferative response.
Comment: The pattern of fibrotic response to chronic injury varies among the species. When hepatic fibrosis is accompanied by a nodular or non-nodular regenerative response in the liver, it may be considered by some to represent cirrhosis. Classical cirrhosis is rare in rodents in contrast to dogs and humans and cannot reliably or consistently separate from a robust fibrotic reaction with associated nodular regeneration, oval cell proliferation, and bile duct hyperplasia. Such a robust fibrosis can be induced in rodents with prolonged or repeated exposure to certain chemicals (carbon tetrachloride, alcohol, tetrachlorovinphos, diallylphthalate), dietary lipotrope deficiency, or chronic hepatitis secondary to persistent infection (Ward 1997). We see no advantage in calling severe hepatic reaction cirrhosis in contrast to a diagnosis of hepatic fibrosis with an appropriate severity grade. The specific morphological features of this response can easily be addressed in the pathology narrative. It should be noted that hepatocellular neoplasms might arise in severe hepatic fibrosis.
D. Infectious Diseases
Introduction
Infectious diseases of the mouse liver are an important group of conditions that may interfere with toxicology and carcinogenesis studies. The histological changes associated with the diseases described in the following may be recorded using the nomenclature described previously under inflammation and inflammatory cell infiltrates but they are also presented here as a separate category of disease diagnoses to help pathologists diagnose the infections, which can be confirmed by PCR, immunohistochemistry, and other diagnostic studies. Some major infectious diseases are mentioned in the following to separate these background lesions from xenobiotic induced lesions.
Helicobacter sp. Hepatitis (Figure 84; See Figure 87)
Pathogenesis: Infection by a number of different Helicobacter spp.
Diagnostic features:
- Focal to multifocal areas of necrosis with or without inflammation.
- Silver stain positive helical to rod-shaped bacteria in or adjacent to the lesions, often can be seen between hepatocytes (because organisms are in the bile caniculi).
- Chronic lesions—focal, multifocal, to diffuse may include inflammatory cell foci, hepatocytomegaly, oval cell hyperplasia, and cholangitis.
- Oval cell hyperplasia, focal to diffuse, minimal to marked.
Differential diagnosis:
- Inflammation, focal or multifocal, acute or chronic, unknown etiology.
- Murine norovirus infection.
- Tyzzer’s disease.
Comment: A number of different Helicobacter spp. have been identified that can affect the rodent liver spontaneously (Ward, Anver, et al. 1994; Ward, Fox, et al. 1994; Goto et al. 2000; Zenner 1999). Helicobacter spp. can cause an increase in hepatocellular tumors in certain strains of infected mice, but are also known to generate liver lesions (Ward, Fox, et al. 1994; Tian et al. 2005; Rogers and Fox 2004) and can promote experimental carcinogenesis of the liver (NTP Toxicology and Carcinogenesis Studies of Theophylline 1998; Zenner 1999; Diwan et al. 1997) in rodents. The pathogenicity of the bacteria can vary with strain of bacteria and mouse strain, stock or line.
Helicobacter hepaticus may cause acute to subchronic minimal to severe lesions in livers of susceptible mice such as A strain, C3H, and BALB/c (Ward, Anver, et al. 1994). Many mouse strains are resistant to liver infection but A strain mice are the most susceptible (Ward, Anver, et al. 1994; Ward, Fox, et al. 1994). Incidental findings of focal or multifocal necrosis in the liver with or without inflammatory cells such as macrophages, lymphocytes, and neutrophils can be seen. In severely affected livers, more severe chronic lesions can be observed. H. hepaticus hepatic lesions are more common in males than females and incidence is increased in mice six months of age or older (Percy and Barthold 2001). H. bilis may also cause mild hepatic lesions.
It is rare for H. hepaticus to cause liver lesions unless the facility and animal room are known to be infected. If susceptible mouse strains are used in research, the more severe diffuse lesions may occur. Mouse infection in two-year carcinogenesis bioassays has complicated interpretation of carcinogenesis studies (Hailey et al. 1998; Stout et al. 2008).
Murine Norovirus Hepatitis (See Figure 73)
Pathogenesis: Infection by murine norovirus.
Diagnostic features:
- Focal, multifocal to diffuse areas of inflammation sometimes with vasculitis in immunodeficient mice.
- Focal to multifocal small areas of inflammation in most lines of mice.
- Inflammatory foci contain macrophages and lymphocytes.
- Immunohistochemistry shows that inflammatory cells, especially macrophages in lesions and Kupffer cells, can be positive for murine norovirus antigens.
Differential diagnosis:
- Helicobacter hepatitis can appear similar but may contain silver positive bacteria.
- Helicobacter hepatitis is more common in A, BALB/c, and C3H strains that are not susceptible to MNV infection.
- Inflammation, focal or multifocal, acute or chronic, unknown etiology.
Comment: Murine norovirus (MNV) may cause severe hepatitis in some lines of immunodeficient mice but only minimal hepatitis or no lesions in most lines of infected immunocompetent mice (Ward et al. 2006). MNV infection is the most common viral infection in mouse colonies today. The implications for interfering in experimental results are not known. It can be assumed that chemicals or infectious agents involving the immune system or liver may be influenced by MNV infection.
Mouse Hepatitis Virus Hepatitis (See Figures 63, 64, and 70)
Pathogenesis: Infection by mouse hepatitis virus affecting hepatocytes, endothelium, and macrophages (Kupffer cells).
Diagnostic features:
- Focal or multifocal hepatic necrosis.
- Multinucleated cells (syncytia) from hepatocytes, endothelium, and macrophages.
- MHV lesions in other tissues, including peritoneum, CNS, blood vessels.
- Chronic hepatitis and postnecrotic cirrhosis in immunodeficient mice.
Differential diagnosis:
- Necrosis caused by toxins.
- Necrosis caused by murine norovirus.
- Necrosis caused by Helicobacter sp.
- Necrosis caused by C. piliforme.
Comment: Mouse hepatitis virus (MHV), a coronavirus, infects hepatocytes, endothelium, and macrophages. In mouse liver, virus strains may have different pathogenicities in the various mouse strains and in mice of different ages (Percy and Barthold 2007). Focal and multifocal necrosis is seen with multinucleated (syncytial) hepatocytes, endothelium, and macrophages. Immunodeficient mice may develop a chronic persistent infection with chronic lesions in liver (Ward, Collins, and Parker 1977).
Clinical MHV infection may be most commonly seen in young mice. Adult mice may have serum antibodies but no clinical signs and few, if any, histopathologic lesions. Some cases show lesions in liver only. MHV is one of the most prevalent murine viruses in the United States and Europe (Homberger 1996) but appears less common today.
Tyzzer’s Disease (Clostridium piliforme Infection)
Pathogenesis: Infection by Clostridium piliforme (Bacillus piliforme).
Diagnostic features:
- Focal or multifocal necrosis, coagulation to caseous with neutrophilic infiltration.
- Intrahepatocyte bundles of long basophilic bacilli.
- Bacteria seen in Warthin-Starry, Giemsa stains, or by the PAS method.
Differential diagnosis:
- Necrosis, of other known causes or unknown causes.
Comment: Named after Ernest Tyzzer who first described it in a colony of Japanese walzing mice (Fox et al. 2002). Clostridium piliforme (Bacillus piliformis) is a long, thin, spore forming (intracellular) bacterium. Rare lesion with sudden death, with or without diarrhea. Often infection of the colon with dissemination to the liver (focal hepatitis) and occasionally heart (myocarditis). Special stains (e.g., Giemsa or Warthin-Starry Silver stain) can reveal intracytoplasmic filamentous bacteria.
Tyzzer’s disease is rare in rodents. It is usually sporadic. The gerbil is known to be extremely susceptible to infection (Fox et al. 2002).
Gross lesions can include hepatomegaly, focal necrosis, single or multifocal, small or large lesions sometimes associated with lesions in other tissues, especially intestines (Percy and Barthold 2007). Multiple foci of necrosis (coagulative necrosis) and/or multifocal necrotizing hepatitis can be observed microscopically.
E. Vascular Lesions
Introduction
The liver has a dual blood supply consisting of a relatively major (about 75%) venous (portal) supply via the hepatic portal vein, which carries venous blood that is largely depleted of oxygen, and relatively minor (about 25%) arterial blood supply, via the hepatic artery. The portal blood contains toxic materials absorbed in the intestine, and therefore the liver is the first tissue to be exposed to toxic substances that have been absorbed through the gastro-intestinal tract.
Within the hepatic parenchyma, the hepatocytes are in intimate contact with the sinusoidal capillaries, which are carrying the mixture of blood originating from ramifications of the portal vein and hepatic artery to the central vein. The sinusoids are lined by modified endothelial cells containing fenestrations, which allow passage of lipoproteins and other large molecules but provide a barrier to blood cells. Kupffer cells reside in the lumen of the sinusoids and are anchored to their wall.
The morphological aspect of the hepatic pathology during circulatory disorders depends on the location of the vascular structure being affected (i.e., lobular sinusoids, the outflow hepatic vein, or the inflow portal vein).
Congestion (Figure 85)
Synonym: Chronic passive congestion.
Pathogenesis: Circulatory failure, typically right-sided heart failure.
Diagnostic features:
- Increase prominence (number) of erythrocytes in the capillary bed or larger vessels of an organ.
- No appreciable distention (angiectasis) of the vessel wall.
- Diagnosis often correlates with gross observation (e.g., reddened, darkened focus).
- May be associated with centrilobular necrosis.
Differential diagnosis:
- Angiectasis—dilated vascular spaces lined by endothelial cells; dilated vascular channels and spaces frequently contain erythrocytes.
- Massive necrosis.
- Hemorrhage-irregular patchy lakes of blood not contained within defined vascular channels.
- Autolysis-altered cellular texture and loss of staining intensity.
Comment: Congestion may result from circulatory disturbance such as right-sided heart failure and is usually associated with necrosis of the centrilobular areas (Burt, Portmann, and MacSween 2002). Presence of blood in hepatic sinusoids seen in animals that die or in situations where there is incomplete exsanguination should not be diagnosed as congestion. If it is absolutely necessary to record such findings, it is important to qualify the blood stasis in the liver as passive congestion.
Angiectasis (Figures 86 and 87)
Synonyms: Peliosis hepatis, telangiectasis, sinusoidal dilation.
Pathogenesis: Perturbations in blood flow and/or drainage; weakening of sinusoidal walls.
Diagnostic features:
- Macroscopically—seen on the surface as blood-filled, thin-walled cavities projecting above the surface (Bannasch et al. 1997).
- Microscopically—There are two morphological types, as follows:
- Cystic (‘‘Phlebectatic’’)—Focal dilation (distension) of endothelial lined channels (photographic presentation contributed by Hardisty et al. 2007). Can be an isolated lesion or have a multicentric form. The lacunae are densely packed with blood and are coated by a single layer of endothelium and separated from one another by cords of liver parenchyma (Bannasch, Wayss, and Zerban 1997). The endothelial cells appear to be unaltered, and there is no increase of mitotic figures. The tissue adjacent to the dilated sinusoids is well preserved and free of necrotic cells.
- Cavernous (‘‘Parenchymal’’)—The peliotic cysts are not, or are only partially, lined by endothelium. Thus, the cysts involve not only the sinusoidal lumen, but also the space of Disse, and the blood comes in direct contact with the neighboring parenchymal cells. The parenchyma undergoes focal necrosis without a zonal distribution. This lesion is much less likely to be preneoplastic and a few toxins have been shown to induce the lesion. When endothelial lining is absent, the term Peliosis Hepatis instead of Angiectasis is indicated.
Differential diagnosis:
- Hemangioma—expansile structure lined by flattened endothelial cells; may be associated with parenchymal compression.
- Cystic degeneration (Spongiosis hepatis)—cavities are not filled with blood but with a finely flocculent acidophilic material.
Comment: Angiectasis is a cystic or cavernous widening of the liver sinusoids that can occur in a variety of pathological insults. In human, sinusoidal dilatation has been reported following hypoxia or hyperperfusion as a result of right-sided heart failure, thrombosis of hepatic veins, amyloid deposition, granulomatous, or neoplastic disease (Greaves and Faccini 1992; Bruguera et al. 1978). Although these lesions can also occur spontaneously in different rodents with different diseases or with certain neoplasms causing hemodynamic changes, it can also be induced by different compounds. Focal sinusoidal dilatation and peliosis hepatis have been observed in the rodent liver after treatment with nitrosamines, pyrrolizidine alkaloids or glucocorticoids (Greaves 2007; Ruebner, Watanabe, and Wand 1970; Ungar 1986; Wolstenholme and Gardner 1950). Altered hemodynamic and changes in the hepatic microcirculation have been proposed to be of importance in the pathogenesis of sinusoidal dilatation (Slehria et al. 2002). Subcapsular sinusoidal dilatation can also be found a postmortem finding in rats (Kimura and Abe 1994).
Angiectasis is defined by multiple blood-filled cystic spaces of different size and shape occurs after suspected loss or weakening of sinusoidal walls and/or supporting tissue. The two subtypes may occur in combination. The cystic spaces are devoid of endothelial lining in the cavernous subtype (‘‘parenchymal’’) although controversy still exists (Greaves 2007; Edwards, Colombo, and Greaves 2002). Angiectasis can be found in aged rats (Lee 1983). However, these lesions can also be induced in both rats and mice after viral infection (Bergs and Scotti 1967) or exposure to certain drugs or chemicals (Mendenhall and Chedid 1980; Husztik, Lazar, and Szabo 1984).
Angiectasis has also been reported in animals or humans infected with Bartonella spp. (Wong et al. 2001; Breitschwerdt and Kordick 2000) and it has been associated with a number of diseases in humans as well as administration of anabolic steroids and oral contraceptives (Naeim, Cooper, and Semion 1973; Zimmerman 1998; Tsokos and Erbersdobler 2005).
Angiectasis may refer to a vascular lesion formed by dilatation of a group of small blood vessels. It can be observed in transgenic mouse models (Srinivasan et al. 2003; Bourdeau et al. 2001) or related to xenobiotic administration in rats and mice (Robison et al. 1984; Kim et al. 2004). It can be found as an incidental finding in aging mice and is sometimes associated with hepatocellular neoplasms (Harada et al. 1996, 1999). Angiectasis can be chemically induced (Bannasch, Wayss, and Zerban 1997) and has been suggested to be preneoplastic in some animal models.
Thrombosis (Figure 88)
Pathogenesis: Activation of the coagulation system associated with arteritis or phlebitis or secondary to atrial thrombosis.
Diagnostic features:
- It is characterized by the formation of a thrombus within the lumen of a blood vessel, like sinusoids and central veins.
- Amorphous mass attached to the endothelium or free within the blood vessel lumen (due to plane of section).
- Contains fibrin, platelets, and entrapped blood cells.
- Damaged endothelium can be seen.
- May occur with histiocytic sarcoma in rats or mice.
Differential diagnosis:
- Postmortem clot.
- Necrotic area of tissue—loss of microscopic tissue structure and stain affinity; pale eosinophilic staining, and absence of nuclear detail.
Comment: There are several potential mechanisms leading to liver thrombosis. Activation of the coagulation system associated with fibrin deposits and hypoxia located in the centrilobular sinusoids was reported to occur in the livers of rats exposed to monocrotaline (MCT) (Copple et al. 2002). It was suggested that the fibrin thrombi were formed following chemical-induced hepatic endothelial cell damage. In studying an endotoxin-exposure model, it was suggested that the noted focal and random hepatocellular necrosis was caused by circulatory disturbances due to fibrin thrombi in clusters of adjacent sinusoids. Using a rat model of 2-butoxyethanol induced hemolytic anemia associated with systemic thrombosis, fibrin thrombi were noted in the central vein and sinusoids of the liver, in addition to the presence of thrombi seen in several other organs (Ramot et al. 2007).
Infarction (Figures 89 and 90)
Pathogenesis: Interruption of blood flow in a major vessel. Torsion of hepatic lobe.
Diagnostic features:
- Extensive area of necrosis may be associated with inflammation.
- The necrosis does not have acinar pattern.
Differential diagnosis:
- Hepatocellular necrosis resulting from direct toxicity may have a diffuse or lobular distribution but also may be accompanied by infarction; not mutually exclusive.
Comment: Aside from torsion of liver lobes, which can occur spontaneously in rodents, infarction is a very rare lesion that can be induced only under very specific experimental conditions. The combined injection in mice of NG-monomethyl-L-arginine and aspirin after lipopolysaccharide exposure resulted in significant hepatocellular enzyme release, characterized histologically by intravascular thrombosis with diffuse infarction and necrosis (Harbrecht et al. 1994). Intraperitoneal injections in rats of vasoconstrictor xenobiotics such as phenylephrine produced infarcts of the spleen regularly and infarcts of the liver occasionally (Levine and Sowinski 1985). Isolated perfusion with 1.0 g/kg of the cytotoxic xenobiotic 5-FU or hyperthermia of 41 degrees C x 10 min resulted in 90% to 100% mortality in rats, with extensive, patchy necrosis, and infarction on histologic examination (Miyazaki et al. 1983).
Endothelial Cell Hypertrophy/Karyomegaly3 (Figure 91)
Synonyms: Endothelial cell enlargement, cytomegaly.
___________________________________
3 The examples provided were confirmed with special stains (not shown).
However, based solely on H&E staining, a diagnosis of ‘‘sinusoidal cell hypertrophy/karyomegaly’’ is appropriate.
Introduction: This is a relatively new diagnostic entity. Because of the difficulty of identifying specific sinusoidal cell types from H&E-stained sections, definitive diagnosis of endothelial cell hypertrophy/karyomegaly requires confirmation using special stains.
Pathogenesis: Continued DNA synthesis and cell cycle arrest following exposure to certain xenobiotics.
Diagnostic features:
- Irregularly shaped karyomegalic nuclei.
- Nuclei and cells appear larger than normal.
- Secondary changes related to the endothelial cell damage and/or obstruction of the sinusoids leading to local ischemia may be seen. Such changes include sinusoidal congestion, hemorrhage, thrombi formation, hepatocytic fatty change/degeneration, and necrosis (Lailach et al. 1977; Nyska et al. 2002).
Differential diagnosis:
- Endothelial cell hyperplasia characterized by increased number of endothelial cells.
- Hepatocytic cytomegaly and karyomegaly.
- Anisokaryosis—variation in the size of nuclei and/or binuclearity admixed with diploid appearing hepatocyte nuclei.
- Reactive Kupffer cells—enlarged histiocytic cells with visible cytoplasm lining sinusoids.
- Histiocytic sarcoma—hepatic parenchyma infiltrated and replaced by collections of histiocytic cells that can be pleomorphic.
Comment: In a study of monocrotaline (Wilson et al. 2000) it was suggested that the endothelial karyomegaly was the result of continued DNA synthesis and concentration-dependent cell cycle arrest. The exposed cells undergo a process of multiplication of chromosomal copies, defined as endopolyploidy, with nuclear and cytoplasmic gigantism. A direct correlation between cytoplasmic volume and nuclear DNA content was suggested.
A similar pathogenesis was suggested in the case of riddelliine-induced endothelial cytomegaly and karyomegaly (Nyska et al. 2002). Administration of riddelliine, a naturally occurring pyrrolizidine alkaloid, to rats results in cytomegaly and karyomegaly of hepatic endothelial cells as one of its pleotrophic responses to cell-specific cytotoxicity. A metabolite of this pyrrolizidine alkaloid is believed to directly interact with endothelial cell DNA.
Sometimes this change is interpreted as prominent Kupffer cells or Kupffer cell hypertrophy on H&E sections and warrants further characterization for confirmation of the endothelial cell origin. Immunohistochemical stains and electron microscopy can be used to identify the cell type involved.
Hyperplasia, Endothelial
Synonyms: Endothelial cell hyperplasia, angiomatous hyperplasia.
Pathogenesis: Proliferation of normally present sinusoidal lining endothelial cells without sinusoidal dilation.
Diagnostic features:
- Focal, located to the portal triad, consisting of increased number of capillaries.
- Blood may not be present within the capillaries.
- Absence of supporting tissue.
- May have mitotic figures and nuclear atypia.
Differential diagnosis:
- Hemangioma—expansile structure lined by flattened endothelial cells; may be associated with parenchymal compression.
- Angiectasis—dilated vascular spaces lined by endothelial cells; dilated vascular channels and spaces frequently contain erythrocytes.
Comment: Endothelial derivation and proliferation can be confirmed by dual immunostaining for CD31/KI-67 (Ohnishi et al. 2007). Comparative endothelial cell kinetic studies in human, mice, and rats indicated that the labeling index (LI) in the male and female B6C3F1 mouse liver was significantly higher (p < .01) compared to the LI in male and female rat and human liver, and the LI in the male and female rat liver was significantly greater (p < .05) than the LI in human liver. It was suggested that the increased rate of spontaneous hemangiosarcoma formation in mice may be related to the increased proliferation rate of endothelial cells normally present in the B6C3F1 strain of mice compared to rats and humans (Ohnishi et al. 2007).
F. Non-Neoplastic Proliferative Lesions
Introduction
A variety of non-neoplastic proliferative lesions of different origin(s) occurs spontaneously in the liver of rodents and may also be induced by treatment with chemicals. Incidences and morphological characteristics vary considerably by animal species, strain, and sex. Some of these lesions might be regarded as pre-neoplastic alterations.
As a non-neoplastic proliferative response, increased hepatocyte mitoses (Figure 92) above normal background levels or increased above what is seen in control animals can occur in rodent livers. Causes vary from physiological responses such as during early growth, during pregnancy, and following partial hepatectomy to post-necrotic repair. This change may be diagnosed as cytologic alteration or mitotic alteration with a description in the pathology narrative. In some laboratories diagnosis of ‘‘increased mitoses’’ is used.
Focus of Cellular Alteration
Introduction: Foci of cellular alteration are common in rodent studies greater than duration of twelve months and may be seen in short duration toxicity studies following exposure to certain xenobiotics. Foci of cellular alteration can be identified by special stains. In H&E-stained slides they may be subclassified based on the predominant cell type present. Diagnosis of the mixed cell subtype of altered hepatic focus varies among different laboratories. One viewpoint defines a mixed focus of cellular alteration as consisting of a combination of basophilic, vacuolated, eosinophilic, and/or clear cell hepatocytes without a predominant cell type. An alternative viewpoint defines a mixed cell focus as containing any two phenotypes of cells in approximately a 50%/50% proportion. Others regard a ‘‘true’’ mixed focus as one that contains clearly identified basophilic and eosinophilic cells regardless of the proportions of each. Because of this diverse set of diagnostic opinions regarding focus subtypes, the pathologist is encouraged to describe the morphological features of documented foci in detail, especially if they are altered by treatment.
Synonyms: Areas of cellular alteration; focus of altered hepatocytes; hyperplastic focus; preneoplastic focus; enzyme altered focus; phenotypically altered focus.
Pathogenesis: A localized proliferation of hepatocytes phenotypically different from surrounding hepatocyte parenchyma.
Diagnostic features:
- May occasionally be observed grossly as small white foci on the liver surface, but not round nodules.
- Size may range from less than one lobule to multiple lobules in diameter.
- Circular or ovoid shape; irregular formed foci may occur.
- Distinguished into types of foci by virtue of tinctorial variation, size of hepatocytes, and textural appearances from surrounding parenchyma.
- May be subclassified based on predominant cell type. The fact that 80% of the focus is composed of one morphologic cell type (basophilic, eosinophilic, etc.) or a mixed cell type.
- Normally no or only minimal compression of the surrounding liver tissue.
- Liver plates merge imperceptible with surrounding hepatic parenchyma; nevertheless, foci are sharply demarcated from the adjacent normal hepatocytes by the appearance and staining reaction of its cells.
- Lobular architecture preserved.
- Portal areas and central veins not present in small foci but can be seen in larger foci.
- Sinusoids within focus may be compressed, so typical parenchymal plates are difficult to detect.
- Tortuous hepatic cords may occur due to increased number of cells.
- Size of cells and cytoplasmic tinctorial variation depend on type of focus.
- Normally absence of cellular atypia.
- Cystic degeneration (spongiosis hepatis) and angiectasis (peliosis hepatis) may occur within foci of altered cells.
- Cytoplasmic lipid may be present.
- Intracytoplasmic inclusions of various types may be present.
Basophilic (Figures 93–97)
Basophilic, diffuse (Figures 93 and 94).
- Hepatocytes of normal size or slightly enlarged with homogeneously staining cytoplasmic basophilia due to abundant free ribosomes.
- Cells may be pleomorphic with enlarged vesiculated nuclei and prominent nucleoli.
- Dissociation of cells may occur.
- Mitotic figures may occur.
Basophilic, tigroid (Figure 95).
- Cells are usually smaller; enlarged cells may occur.
- Cells are often arranged as tortuous cords.
- Cells display large abundant basophilic bodies often arranged in clumps or long bands with striped pattern in paranuclear or peripheral regions of cytoplasm (due to increased rough endoplasmic reticulum).
- Mitotic rate may be increased.
Basophilic, NOS (Figure 96).
- Foci that are not clearly tigroid or diffusely basophilic.
- Peliosis or spongiosis may occur within these foci.
Basophilic (no further classification in mice) (Figure 97).
- Consist of cells larger or smaller than normal hepatocytes, in general they are smaller.
- Cytoplasm exhibits distinct basophilia due to free ribosomes or rough endoplasic reticulum.
- Often cells contain obvious glycogen.
- Intracytoplasmic basophilic clumps with relatively clear intervening cytoplasm or the cytoplasmic basophilia may be distributed homogeneously.
- Vascular pseudo-invasion may be present.
- Eosinophilic cytoplasmic inclusions may be found occasionally within hepatocytes.
Eosinophilic (Figures 98–100)
Synonyms: Acidophilic, ground glass.
- Composed of usually enlarged, polygonal hepatocytes with acidophilic staining cytoplasm.
- Some clear cells may be present.
- Cytoplasm is distinctly granular and pale pink, intensely eosinophilic, or shows ground-glass appearance.
- Nuclei often enlarged; nucleoli may be prominent and centrally located.
- Eosinophilia may result from an increase in smooth endoplasmic reticulum, peroxisome, or mitochondria (rats, mice).
- Store glycogen in excess.
Mixed Cell (Figures 101–104)
Synonyms: Basophilic/eosinophilic mixed.
Heterogeneous focus consisting of a combination of cell types (see Introduction above).
Clear Cell (Figures 105–107)
- Composed of normal-sized or enlarged cells with distinct cytoplasmic clear spaces.
- Some eosinophilic or basophilic cells may be present.
- Nuclei are often small and dense, prominent and centrally located, sometimes exhibiting increased volume.
- Store glycogen in excess.
- Cell membranes may appear prominent.
Amphophilic (in Rats and Mice) (Figure 108)
- Hepatocyte cytoplasm having an affinity both for acid and for basic dyes.
- Large cells with homogeneous, granular intensely eosinophilic cytoplasm, and randomly scattered faint basophilia.
- Nuclei slightly enlarged.
- Poor in or free of glycogen.
- Amphophilic (basophilic and eosinophilic) cytoplasm due to a proliferation of both mitochondria and rough endoplasmic reticulum (mitochondrial-RER complex).
- Usually less frequently observed in rats and mice compared to other foci.
Differential diagnosis:
- Hyperplasia, hepatocellular, regenerative—evidence of prior or ongoing hepatocellular damage.
- Hyperplasia, hepatocellular, non-regenerative—usually seen as a single large nodule of hyperplasia without concomitant evidence of hepatocellular damage or phenotypical alteration. Rare in rats and mice.
- Adenoma, hepatocellular—loss of normal lobular architecture with irregular growth pattern. Liver plates often impinge perpendicular or obliquely on the surrounding parenchyma. Distinct compression of adjacent normal hepatocytes is present.
- Carcinoma, hepatocellular—distinct trabecular, adenoid or poorly differentiated growth is present. Lobular and plate architecture is not maintained. Cellular atypia and invasive growth may be present.
Comments: Foci of cellular alteration represent a localized proliferation of hepatocytes that are phenotypically different from surrounding hepatocyte parenchyma. They are subclassified based upon phenotypic and tinctorial features. Foci of cellular alteration can occur spontaneously in aged rats and other rodents and can be induced by chemical treatment. The incidence, size, and/or multiplicity of foci are usually increased and time to development usually decreased after administration of hepatocarcinogens (Hanigan, Winkler, and Drinkwater 1993; Frith, Ward, and Turusov 1994; Bannasch and Zerban 1990; Moore, Thamavit, and Bannasch 1996). Foci of cellular alteration are not necessarily preneoplastic. Foci of cellular alteration may have prominent fat deposition and characteristic features of cystic degeneration and angiectasis (See B-focus with spongiosis; Figure 96).
Foci can be subclassified based on the predominant cell type. If no single cell type comprises at least 80% of a given focus, it should be classified as mixed. Mostly these mixed foci consist of both basophilic and eosinophilic/clear type cells. In these foci, it is not clear what is the predominant phenotype and are therefore indicated as ‘‘mixed.’’
Species and strain differences occur in the prevalence of these foci. It is not uncommon for a focus predominantly of one cell type, however, to have a small number of a different type.
Mixture of eosinophilic and clear cells can be classified into either eosinophilic or clear cell focus in accordance with proportion of clear cells. Mixture of amphophilic and other phenotypes has never been observed in rodents.
Usually, amphophilic foci are less frequently observed in rats and mice compared to other foci.
A number of models have linked specific types of foci of cellular alteration with carcinogenesis (Mahon 1989). The nitrosomorpholine model is linked with eosinophilic and clear cell foci as precursors. Aflatoxin is linked with basophilic foci as a tumor precursor (Bannasch, Zerban, and Hacker 1985). It was also reported that hepatocarcinogenesis was associated with increase of basophilic or amphophilic foci (Goodman et al. 1994). Although age-related eosinophilic or tigroid basophilic foci were not associated with exposure to hepatocarcinogens, in hamsters, treatment with nitrosamines or other carcinogens caused a variety of foci including basophilic foci (Frith, Ward, and Turusov 1994; Moore, Thamavit, and Bannasch 1996). As some foci may be potential precursors of neoplastic formation, careful identification of altered foci is warranted (Maronpot et al. 1989). Although induced by carcinogens, foci of cellular alteration can be found as non-neoplastic end stage lesions and not all foci can be related to carcinogens (Peraino et al. 1984; Harada, Maronpot, Morris, Stitzel, et al. 1989; Harada, Maronpot, Morris, and Boorman, 1989; Squire 1989; Schulte-Hermann et al. 1989). Most importantly, types of foci in controls should be compared to those found in treated animals. Some pathologists regard vacuolated foci as focal fatty change and do not consider them a subtype of focus of cellular alteration.
Hyperplasia, Hepatocellular, Non-Regenerative (Figures 109 and 110)
Synonyms: Hyperplasia, hepatocellular, focal hepatocellular hyperplasia.
Pathogenesis: A spontaneous or treatment-associated proliferative collection of hepatocytes spanning several lobules and without evidence of prior hepatic damage.
Diagnostic features:
- A relatively large lesion that is often greater than several adjacent lobules and is occasionally accompanied by angiectasis and/or spongiosis hepatis (cystic degeneration).
- Comprised of slightly enlarged hepatocytes.
- Hepatocytes are tinctorially similar to surrounding parenchyma.
- The liver plates in the lesion tend to merge with the adjacent hepatic parenchyma.
- May be minimal to mild compression of adjacent hepatic parenchyma.
- Lobular architecture is maintained.
- Portal triads and central veins are present.
- When accompanied by angiectasis/spongiosis hepatis, hepatic cords may be distorted.
Differential diagnosis:
- Focus of cellular alteration—phenotypical or tinctorial variation is present. Generally not associated with chronic liver damage, though foci of cellular alteration may occur in damaged liver.
- Adenoma, hepatocellular—loss of normal lobular architecture with irregular growth pattern. Liver plates often impinge perpendicular or obliquely on the surrounding parenchyma. Distinct compression is present.
- Carcinoma, hepatocellular—distinct trabecular, adenoid, or poorly differentiated growth is present. Lobular and plate architecture is not maintained. Cellular atypia and invasive growth may be present.
- Hyperplasia, hepatocellular (regenerative)—evidence of ongoing or prior hepatocellular damage, fibrosis, or a history of exposure to a known hepatotoxicant. Lobular architecture usually distorted.
Comment: Non-regenerative hepatocellular hyperplasia is rare in rodents. It may occur spontaneously or be treatment-associated and consists of a proliferative collection of hepatocytes spanning several lobules and without evidence of prior hepatic damage. This lesion is not associated with any evidence of existing or prior hepatocellular injury. Diagnostic difficulties occur when preneoplastic foci and hepatocellular adenomas occur in the same liver sections of older rodents. This lesion may be similar to that of hepatic nodular hyperplasia in dogs.
There are basically two variations of non-regenerative hepatocellular hyperplasia. One is relatively smaller and is accompanied by angiectasis and/or spongiosis hepatis and the other tends to be larger than several lobules. The former occurs in both sexes and the latter predominantly in untreated female control F344 rats but occasionally reported in treated rats (Tasaki et al. 2008; Hailey et al. 2005; Bach et al. 2010). When present near the capsular surface, this type of nodular hyperplasia may be evident grossly as a raised area. The proliferating cell nuclear antigen (PCNA) labeling index in these nodules is increased in comparison with surrounding parenchyma and the lesion is glutathione S-transferase placental form (GSTP) immunonegative.
Very early non-regenerative hyperplasia may be the size of small foci of cellular alteration and are identified by their altered growth pattern and tinctorial similarity to surrounding parenchyma (see Figure 109).
Hyperplasia, Hepatocellular, Regenerative (Figures 111–113)
Synonyms: Hyperplasia, hepatocellular; hyperplasia, regenerative; hyperplasia, nodular; regeneration, nodular.
Pathogenesis: A nodular regenerative response to prior or continuous hepatocellular damage.
Diagnostic features:
- Focal or multifocal (nodular) appearance.
- Lesion may reach several millimeters in diameter.
- Spherical proliferation may be accompanied by slight encapsulation.
- Compression of surrounding liver parenchyma often occurs.
- Normal lobular architecture usually present but may be distorted.
- Portal triads and central veins may be present.
- Bile duct and oval cell proliferation may be present.
- Hepatocytes appear slightly altered, but may have slightly basophilic cytoplasm or prominent nucleoli.
- Increased mitotic index may be observed.
- Evidence of prior or ongoing hepatocellular damage, such as apoptosis/necrosis, chronic inflammation, chronic congestion, fibrosis, cirrhosis, or a known cause of toxicity.
- Lesions in rats tend to be more nodular than in mice.
Differential diagnosis:
- Focus of cellular alteration—tinctorial variation is present. Generally not associated with chronic liver damage, though foci of cellular alteration may occur in damaged liver.
- Adenoma, hepatocellular—loss of normal lobular architecture with irregular growth pattern. Liver plates often impinge perpendicular or obliquely on the surrounding parenchyma. Distinct compression is present.
- Carcinoma, hepatocellular—distinct trabecular, adenoid, or poorly differentiated growth is present. Lobular and plate architecture is not maintained. Cellular atypia and invasive growth may be present.
- Hyperplasia, hepatocellular (non-regenerative)—no history or evidence of hepatocellular damage.
Comment: These lesions are considered to represent a regenerative response to prior or continuous hepatocellular damage. A history of exposure to a hepatotoxicant and the presence of multiple nodules of regeneration that maintain a lobular but usually distorted architecture makes diagnosis more convincing. Diagnostic difficulties occur when preneoplastic foci and hepatocellular adenomas occur in the same liver sections of older rodents or in livers with many induced foci and tumors.
In livers with partial hepatectomy (PH), the pattern of hyperplasia at twenty-four to seventy-two hours post-surgery is diffuse hepatocyte hyperplasia with many mitotic figures and no evidence of liver toxicity. Although this response is also hyperplasia (hepatocellular, regenerative), it is not to be confused with the nodular hepatic response to toxic damage to hepatocytes. After ninety-six hours, the liver may be almost normal histologically. In mice however, chronic biliary lesions may be seen in the liver after PH.
Hypertrophy/Hyperplasia, Kupffer Cell (Figures 114 and 115)
Synonyms: Kupffer cell proliferation; histiocytosis, focal or diffuse.
Pathogenesis: Following phagocytosis of foreign material, estrogen treatment, inflammatory conditions, and response to cytokines. A rare spontaneous finding.
Diagnostic features:
- Diffuse to multifocal proliferation of oval to spindeloid cells lining sinusoids.
- Cells resemble histiocytes and often contain phagocytic material.
- May form as sheets or nodules.
- Hypertrophy can occur without hyperplasia and vice versa.
Differential diagnosis:
- Infiltration, mononuclear—well demarcated; usually solitary; associated with cell necrosis.
- Histiocytic sarcoma—diffuse and irregular proliferation of histiocytic cells throughout the liver. Usually associated with destruction of hepatic parenchyma. Occasional multinucleated giant cells may be present.
- Hyperplasia, oval cell—consists of a single or double row of oval cells sometimes forming incomplete (pseudo-) duct-like structure. Cells are usually uniform in size and shape and have scant pale basophilic cytoplasm and round to oval nuclei.
Comment: Rare as a spontaneous finding, hyperplasia of Kupffer cells may be seen following phagocytosis of foreign material and as a consequence of estrogen treatment and in inflammatory conditions. It can be induced by cytokines. Hypertrophy and hyperplasia often accompany each other. The hypertrophy of normal Kupffer cells gives the impression of presence of more Kupffer cells since they are difficult to visualize in normal livers.
Hyperplasia, Ito Cell (Figures 116–118)
Synonyms: Stellate cell; perisinusoidal cell; fat-storing perisinusoidal cell.
Pathogenesis: Proliferation of fat-storing perisinusoidal cells.
Diagnostic features:
- Focal or diffuse proliferation of Ito cells.
- May grow in sheets, clusters, or along cords of hepatocytes.
- Cells vary in size and shape and are vacuolated.
- Multiple cytoplasmic fat droplets of different size occur.
- The nuclei are ovoid or round and may be indented by cytoplasmic lipid droplets.
- Modest amount of collagenous matrix may be present.
Differential diagnosis:
- Fatty change/Lipidosis—the cytoplasm of fat cells may be clearer than that of Ito cells.
- Ito cell tumor—larger and more extensive than hyperplasia. Partially distinct compression of adjacent hepatic parenchyma.
Comment: Ito cell hyperplasia is extremely rare and occurs predominantly in mice. It arises from fat-storing perisinusoidal cells, better known as Ito cells (Dixon et al. 1994; Enzan 1985; Tillmann et al. 1997). The biological behavior of the lesion is not well established. There appears to be a continuum with Ito cell tumor (see the following), which may be just an exaggerated and sometimes more localized form of Ito cell hyperplasia.
Bile Duct Hyperplasia (Figures 119–122)
Pathogenesis: A spontaneous change in portal areas of older animals; may be induced or exacerbated by treatment.
Diagnostic feature:
- Increased number of small bile ducts arising in portal region.
- May not involve all portal areas.
- May be associated with periductular fibrosis and periductular cell infiltration.
- Biliary epithelium is well differentiated, forming normal ducts.
- Biliary epithelium may show degenerative or atrophic changes.
- May be associated with oval cell hyperplasia.
- May contain mucous metaplasia or hyalinosis in specific situations in mice.
- Cystic form.
- Focal bile duct proliferation with cystic dilation of ducts may occur.
- Usually, acini lined by flattened epithelium.
Differential diagnosis:
- Cholangioma—well demarcated; usually solitary; acini lined by cuboidal epithelium.
- Cholangiofibrosis—central portions become atrophic, collagenized, and avascular whereas the active proliferating portion is at the periphery; mucus production is common.
- Cholangiocarcinoma—invasive growth into surrounding hepatic tissue or vessels; cell is pleomorphic; mucus production.
- Oval cell hyperplasia—consists of a single or double row of oval cells forming incomplete duct-like structures. Cells usually uniform with scant basophilic cytoplasm and round or oval nuclei.
Comment: Often associated with evidence of hepatic injury and repair and obstruction of bile flow. Dilation of intrahepatic bile ducts is a spontaneous, age-associated lesion that is more common in rats than in mice.
Cholangiofibrosis (Figures 123–126)
Synonyms: Bile duct adenomatosis; intestinal cell metaplasia; adenofibrosis.
Pathogenesis: Originates from an initial oval cell hyperplasia in response to pronounced hepatic parenchymal necrosis.
Diagnostic features:
- Consists of dilated to cystic bile ducts filled with mucus and cellular debris and surrounded by inflammatory cell infiltrates and connective tissue.
- Glandular epithelium is typically a single layer and varies from flattened to tall columnar hyperbasophilic and pleomorphic cells along with goblet cells and occasional Paneth cells.
- Glandular epithelium, particularly in cystic glands, may be partially lost through degeneration resulting in crescent shaped structures.
- Central portions of large lesions may become sclerotic with only remnants of biliary epithelium suggesting regression.
- Lesions may be limited to small foci but may occupy large interconnecting areas of a lobe without markedly disturbing the lobe outline.
- Lesion growth typically involves contraction with retraction of surrounding parenchyma. Markedly dilated or cystic mucus-filled glands along the liver capsule may protrude above the lobe outline.
- Older lesions may be shrunken from the liver surface and appear as scars.
- Regenerative hepatocellular hyperplasia may be present when there is extensive parenchymal involvement.
Differential diagnosis:
- Cholangiocarcinoma—solid sheets, trabeculae, or nests of closely packed biliary cells largely displacing hepatic parenchyma. Intestinal metaplasia is not a prominent feature. Glandular dilation is absent or minimal.
- Hyperplasia, bile duct—multiple small bile ducts arising in portal region. Biliary epithelium is well differentiated, forming more normal appearing ducts, with minimal glandular dilation in some cases.
Comment: This lesion is an inflammatory, proliferative, and metaplastic reaction involving bile duct epithelium and is seen with hepatocellular toxicity caused by xenobiotics such as dioxins, furans, and related chemicals in rats (Bannasch and Zerban 1990; Deschl et al. 1997; Eustis et al. 1990; Kimbrough et al. 1973; Kimbrough and Linder 1974; Sirica 1992; Hailey et al. 2005). The initial reaction following pronounced hepatic parenchymal necrosis is oval cell hyperplasia (Engelhardt 1997).
Cholangiofibrosis is a controversial lesion that has been diagnosed as cholangiocarcinoma especially when there is extensive involvement of the liver. Cholangiofibrosis is not seen as a spontaneous lesion but occurs primarily in rats treated with a variety of xenobiotic agents that are hepatotoxic at high dose. The intestinal cell metaplasia in cholangiofibrosis includes goblet and Paneth cells and columnar cells similar to chief cells identified in H&E-stained paraffin sections and enterochromaffin cells identified by special staining. The metaplastic cells have been confirmed by electron microscopy in some cases. While cholangiofibrosis has been reported to progress to cholangiocarcinoma with malignant features (Bannasch and Zerban, 1990; Sirica 1992), unequivocal metastases have not been confirmed in most cases. This lesion is not observed in humans.
Oval Cell Hyperplasia (Figures 127–129)
Synonyms: Oval cell proliferation; bile ductule cell hyperplasia.
Pathogenesis: Arises from terminal ductule epithelial cells (canal of Hering cells) spontaneously, following liver infections, and secondary to hepatotoxic injury.
Diagnostic features:
- Generally originates from portal areas and is often multifocal.
- Consists of a single or double row of oval to round cells along sinusoids in linear arrays.
- May form a few or many small ductules with streaming into the hepatic parenchyma.
- Formation of incomplete duct-like structures may be present.
- Cells are usually uniform in size and shape and may be fusiform.
- Cells have scant pale basophilic cytoplasm and round or oval nuclei.
- Oval cells express keratin.
Differential diagnosis:
- Hyperplasia, bile duct—several small bile ducts arising in portal region. Biliary epithelium is well differentiated, forming normal ducts.
- Early or mild fibrosis—collagen matrix is evident.
- Inflammation—presence of mononuclear, polymorphonuclear, or connective tissue cells will be present in inflammation.
Comment: Oval cell proliferation is considered to arise from terminal ductule epithelial cells (canal of Hering cells). It is a rare spontaneous lesion in rats. Oval cell hyperplasia can be observed following severe hepatotoxic injury and treatment with hepatocarcinogens. There is often a close relation to the portal tract, although more scattered groups of proliferating oval cells can be seen diffusely throughout the liver following xenobiotic-induced hepatic injury (Engelhardt 1997).
In mice oval cell hyperplasia is a feature of chronic active hepatitis caused by H. hepaticus and H. bilis and is seen following treatment with various hepatocarcinogens. Hyperplastic oval cells occur in association with a high incidence of hepatocellular neoplasms and may play an important role in hepatocarcinogenesis. Some authors support the concept that oval cells may participate in the lineage of hepatocellular and cholangiocellular carcinomas and may serve as hepatic stem cells. Oval cell hyperplasia diagnosis including grade is recommended, even when it is part of a complex set of hepatic changes.
G. Neoplasms
Introduction
The rodent liver is the most common target site of chemical carcinogens (Maronpot et al. 1986; Evans and Lake 1998), perhaps due to its major function as a metabolizing and detoxifying organ for xenobiotics. Rodent hepatocarcinogens are usually hepatotoxins. The chronic toxicity of these toxins may contribute to hepatocarcinogenesis although genotoxic liver carcinogens are often also hepatotoxins. There is over a thirty-year history of experimental induction (Frith and Wiley 1982; Malarkey et al. 1995; Evans et al. 1992; Ward et al. 1983, 1986; Ward, Lynch, and Riggs 1988; Popp 1984) and classification of preneoplastic and neoplastic lesions of the rat and mouse liver in book chapters (Bannasch and Zerban 1990; Brooks and Roe 1985; Greaves and Faccini 1984; Jones and Butler 1978; Ward 1981; Harada et al. 1999; Eustis et al. 1990) and by committee (ILAR 1980) or toxicologic pathology societies (Standardized System of Nomenclature and Diagnostic Criteria [SSNDC] Guides, http://www.toxpath.org/ssndc.asp). Terminology has evolved to the present nomenclature that is also based on many publications on liver carcinogenesis.
There is evidence from experimental studies documenting the regression of proliferative hepatocellular lesions including foci of cellular alteration, hepatocellular adenomas, and hepatocellular carcinomas following cessation of treatment (Maronpot 2009). A dramatic example was reported in mice following cessation of chronic chlordane exposure (Malarkey et al. 1995). Similar experience has been reported in rats and mice in other studies (Lipsky et al. 1984; Greaves, Irisarri, and Monro 1986; Marsman and Popp 1994) as well as in humans (Frémond et al. 1987; McCaughan, Bilous, and Gallagher 1985; Emerson et al. 1980; Steinbrecher et al. 1981). Agents that require continual administration for the stable presence and growth of preneoplastic and neoplastic rodent liver lesions can be categorized as conditional hepatocarcinogens (Maronpot 2009).
Hepatocellular Adenoma (Figures 130-134)
Synonyms: Adenoma, hepatic; adenoma, liver parenchymal cell; hepatoma, benign; tumor, liver cell, benign; hepatoma, benign; type A nodule.
Pathogenesis: Spontaneous and following treatment with hepatotoxins that are carcinogenic xenobiotics; with gene alterations in genetically engineered mice.
Diagnostic features:
- Are often but not always grossly visible as small (1 mm) to large uniformly round nodular lesions.
- Histologically lesions are nodular and compressing of adjacent normal hepatocytes.
- Sharply demarcated from surrounding liver parenchyma that is often compressed. The compression of adjacent normal hepatocytes should be at least on two quadrants of the adenoma.
- Loss of the normal lobular architecture with irregular growth pattern.
- Liver plates often impinge obliquely on surrounding liver parenchyma.
- Hepatocytes with varying size and tinctorial staining pattern occur.
- Adenomas may also be classified morphology by tinctorial and other cytoplasmic characteristics as described for foci of cellular alteration.
- Staining of tumor cells may resemble that of the surrounding liver parenchyma and be classified as amphophilic.
- Usually, single nodules but multiple adenomas may be present.
- Fibrous encapsulation may occur.
- An enveloped portal triad may occasionally be present.
- Mitotic index may be increased.
- Areas of cellular atypia may be present (pleomorphic nuclei, coarsely clumped chromatin, large nucleoli, increased nucleus to cytoplasm ratio, cytoplasmic basophilia, focal attempts at trabeculae formation as in carcinomas).
- Cells within adenomas may reveal signs of degenerative processes such as intracytoplasmic-inclusions, hyaline bodies, or vacuoles.
- Sinusoids may be compressed or ectatic.
- Necrosis is usually absent.
- Pseudoinvasion of blood vessels may be present.
- Lesions may be grossly visible and/or bulge from natural surfaces.
- May occur in livers with foci of cellular alteration and hepatocellular carcinomas.
Differential diagnosis:
- Focus of cellular alteration—liver plates merge imperceptibly with surrounding hepatic parenchyma; normal lobular architecture present.
- Regenerative hyperplasia (in damaged liver)—evidence of prior or ongoing hepatocellular damage; normal lobular architecture is present, albeit distorted.
- Non-regenerative hyperplasia—some evidence of lobular pattern and presence of central veins and portal triads.
- Hepatocellular carcinoma—invasive growth; no clear demarcation; loss of lobular plates, architecture, and/or occurrence of cellular atypia; adenoid, trabecular, or poorly differentiated growth.
Comment: Hepatocellular adenomas occur spontaneously with increased incidence in older rodents and following treatment with hepatotoxins that are carcinogenic xenobiotics (Harada et al. 1999; Eustis et al. 1990; Stinson, Hoover, and Ward 1981). In mice, hepatocellular carcinomas can be seen histologically arising within adenomas of both spontaneous and induced adenomas (Jang et al. 1992). This process is less common in rats. Occasionally large proliferative hepatocellular lesions are observed that are difficult to diagnose. These lesions can compress adjacent parenchyma and/or bulge from the surface. They also have, at least in some areas, normal to slightly distorted lobular architecture with central veins and portal triads. The biologic nature of these lesions is unknown but because of their size and distinct compression, they are sometimes included in the category of hepatocellular adenoma. It is recommended that large lesions of this type with some evidence of lobular architecture and the presence of central veins and portal areas be diagnosed as non-regenerative hepatocellular hyperplasia (described previously in this document).
Hepatocellular Carcinoma (Figures 135–140)
Synonyms: Adenocarcinoma, liver cell; carcinoma, hepatic cell; carcinoma, hepatocellular; carcinoma, liver cell; hepatoma, malignant; hepatocarcinoma; nodule, type B.
Pathogenesis: Spontaneous and following treatment with hepatotoxins that are carcinogenic xenobiotics; with gene alterations in genetically engineered mice.
Diagnostic features:
- Local infiltrating growth and/or lack of distinct demarcation.
- Marked cellular pleomorphism may occur.
- Alterations of tinctorial staining patterns may occur.
- Loss of normal lobular architecture.
- Vascular invasion or metastases may be observed.
- Increased mitotic index possible.
- Bile ducts may be present.
- Hemorrhage, necrosis, and extramedullary hematopoietic foci may be present.
- May occur as a single morphologic type or a combination of them as in the following.
- May metastasize to lung.
Trabecular:
- Composed of well-differentiated hepatocytes forming trabeculae of multiple cell layers.
- The plates alternate with sinusoids.
- Sometimes the sinusoids may be dilated forming blood lakes.
Acinar (synonym: glandular):
- Neoplastic hepatocytes form a generally single layer around a central clear space.
- The acinar structure may vary from tiny to huge cysts.
- Glandular pattern rarely involves more than 50% of the neoplasm.
- Acinar portion is interspersed with areas that have either trabecular or solid architecture.
Solid:
- Cells tend to be poorly differentiated, small, hyperchromatic, and pleomorphic.
- Sometimes predominant cells are spindle shaped.
- Single nuclei or multinucleated giant forms.
- Mitotic figures may be numerous and bizarre.
- The stroma is generally inconspicuous.
- The vascular structures are immature and often thrombosed.
Adenoid (mouse):
- A layer of neoplastic cells surrounds luminal structures.
- Lining cells usually consist of strongly basophilic cuboidal cells.
Differential diagnosis:
- Focus of cellular alteration—liver plates merge imperceptibly with surrounding hepatic parenchyma; normal lobular architecture present.
- Regenerative hyperplasia—evidence of prior or ongoing hepatocyte damage; normal lobular architecture present, albeit distorted; demarcated from surrounding liver tissue.
- Non-regenerative hyperplasia—some evidence of lobular pattern and presence of central veins and portal triads.
- Hepatocellular adenoma—no local invasiveness, less cellular atypia, sharply demarcated from surrounding liver parenchyma. No formation of a trabecular pattern.
- Cholangiocarcinoma—mucus may be present within the adenoid structures. If there is loss of mucus it is difficult to distinguish.
- Hemangiosarcoma—proliferation of atypical endothelial cells forming blood spaces.
Comment: Occur spontaneously with increased incidence in older rodents and following treatment with carcinogenic and hepatotoxic xenobiotics (Bannasch and Zerban 1990; Brooks and Roe 1985; Greaves and Faccini 1984; Jones and Butler 1978; Ward 1981; Harada et al. 1999; Eustis et al. 1990; Popp 1984, 1985; Vesselinovitch, Mihailovich, and Rao 1978). Diagnosis may be modified based on growth pattern (Frith and Wiley 1982).
The trabecular type of hepatocellular carcinoma is the most common form in rats and to a lesser extent in mice. Hepatocellular carcinomas may appear to arise within preexisting adenomas, especially in mice (Figures 139 and 140). While some authors refer to these lesions as focus of carcinoma in adenoma, such lesions should be diagnosed as hepatocellular carcinoma. These carcinomas exhibit the same morphological appearance as the carcinomas described earlier and hint toward a progression of adenoma into carcinoma. Attention should be paid to hemorrhagic areas, where proliferation of endothelial cells or formation of large sinusoidal-like spaces may lead to the erroneous diagnosis of a vascular tumor.
Hepatoblastoma (Figures 141–144)
Synonyms: Tumor, mixed, poorly differentiated.
Pathogenesis: Unknown but origin from liver blastema cells, neoplastic hepatocytes, oval cells, and biliary epithelial cells proposed.
Diagnostic features:
- Well-circumscribed nodule.
- Distinct encapsulation with variable structure may be present.
- Organoid structures are lined by vascular cavities filled with blood.
- The channels are surrounded by several layers of tumor cells.
- The cells are arranged either radially or concentrically forming rosettes, trabeculae, or pseudo-glandular structures.
- The center of the rosettes sometimes contains amphophilic material or small, endothelium-lined vessel.
- Cells are small, strongly basophilic, and elongated. Sometimes they may be more or less eosinophilic and have smaller, rounder, and less hyperchromatic nuclei.
- Numerous mitotic figures are present.
- Areas of large hemorrhage, pigmentation, fibrosis, or necrosis are present.
- Osteoid, bone, squamous differentiation, and extramedullary hematopoietic foci may be present.
Differential diagnosis:
- Cholangiocarcinoma (poorly differentiated)—glandular structures with mucus production are present. Often evidence is present of extensive fibrosis, but not of osteoid, or bone formation.
- Sarcoma, NOS—only mesenchymal structures are present.
- Carcinoma, hepatocellular-only sparse mesenchymal structures are present. Hepatic cell differentiation is obvious.
Comment: Hepatoblastomas consist of organoid structures often oriented around vascular spaces (Harada et al. 1999; Nonoyama et al. 1986, 1988; Turusov, Day, et al. 1973; Turusov, Deringer, et al. 1973). The cells are primitive in appearance of scant, pale basophilic cytoplasm and ovoid hyperchromatic nuclei. They are usually seen with other types of hepatocellular tumors, especially within hepatocellular adenomas. Rare reports of this lesion in rats appear in literature. They are seen in certain strains of mice and have also been induced by certain hepatocarcinogens (Diwan, Ward, and Rice 1989).
Hepatoblastoma is generally seen within or adjacent to hepatocellular neoplasms. In these cases, some preference has been expressed for a single diagnosis of hepatoblastoma, rather than two diagnoses (the hepatoblastoma and the hepatocellular neoplasm). If this preferred convention of using the single diagnosis of hepatoblastoma is not used, then the alternative convention will need to be defined by the pathologist. Hepatoblastomas can have high rates of lung metastases in some experiments.
Cholangioma (Figures 145 and 146)
Synonyms: Adenoma, bile duct; adenoma, biliary; adenoma, cholangiocellular; cholangioma, benign.
Pathogenesis: Proliferation of biliary cells.
Diagnostic features:
- Glandular acini may vary in size and shape.
- Expansively growing with compression.
- Nucleus is round or oval occasionally vesicular with one or two conspicuous nucleoli.
- Cytoplasm is somewhat basophilic.
- Two types can be distinguished:
Simple:
- Generally uniform well-circumscribed neoplasm.
- Acini are lined by a single layer of cuboidal cells varying in size.
- Occasionally the cells are multilayered.
- Sparse vascular stroma may occur.
Cystic:
- Characteristically composed of dilated glandular acini.
- The acini are lined by cuboidal epithelium.
- Papillary structures are occasionally observed, projecting into the lumen of the cysts.
- Clumps of liver cells may be seen between the cysts.
Differential diagnosis:
- Bile duct cyst—lined by flattened epithelium; no expansive growth; not forming acini or papillary structures.
- Hyperplasia, bile duct—multifocal, usually widespread.
- Cholangiofibrosis—central portions become atrophic, collagenized, and avascular whereas the actively proliferating portion is at the periphery; mucus production is common.
- Cholangiocarcinoma—proliferation of atypical cells, invasive growth.
Comment: Cholangioma is rare in control and treated rodents (Bannasch and Zerban 1990; Brooks and Roe 1985; Frith and Ward 1979; Greaves and Faccini 1984; Harada et al. 1999; Jones and Butler 1978; Lewis 1984; Maronpot et al. 1986).
Cholangiocarcinoma (Figures 147 and 148)
Synonyms: Adenocarcinoma, bile duct; adenocarcinoma, cholangiocellular; carcinoma, bile duct; carcinoma, cholangiocellular; cholangioma, malignant.
Pathogenesis: Arise from proliferating cholangial cells.
Diagnostic features:
- Biliary structures are usually glandular but may have solid or papillary areas, may be poor in or free of mucus, and mostly have a minimal connective tissue component.
- Glandular lining cells are hyperbasophilic and have large nuclei and nucleoli but occasionally have clear cytoplasm due to accumulated glycogen.
- Glands lined by single or multilayered cuboidal or cylindrical cells.
- Clear-cut invasion into vascular and lymphatic structures and surrounding parenchyma may be present.
- May appear as a large solid mass.
- Evidence of metastasis or likelihood of metastasis is expected.
Differential diagnosis:
- Cholangiofibrosis—central portions become atrophic, collagenized, and avascular whereas the actively proliferating portion is at the periphery; dilated glands with mucus production and accompanying inflammation is common. Connective tissue response maybe pronounced.
- Cholangioma—no invasion, sparse stroma, more uniform cells, single layer of cuboidal cells, may have cystic glands.
- Carcinoma, hepatocellular (acinar)—no mucus production; contains obvious neoplastic hepatocytes.
Comment: Cholangiomas and cholangiocarcinomas are rarely seen as spontaneous neoplasms in rats and mice but may occur following exposure to hepatotoxic xenobiotics (Eustis et al. 1990; Frith and Ward 1979; Harada et al. 1999; Jones and Butler 1978; Lewis 1984; Narama et al. 2003). A specific phenotype of cholangiocarcinoma with features of cholangiofibrosis consisting of dilated biliary glands, mucus production, intestinal metaplasia, inflammatory cell infiltrates, and fibrosis has been diagnosed in rats treated with a variety of hepatotoxic xenobiotics (Bannasch and Zerban 1990; Bannasch, Brenner, and Zerban 1985; Sirica 1992; Kimbrough and Linder 1974; Maronpot et al. 1986). The distinction between cholangiofibrosis and this phenotype of cholangiocarcinoma is difficult and controversial and is primarily based on extent of liver involvement since unequivocal metastasis is rare.
These rare lesions of cholangiocarcinoma can contain parameters of cholangiofibrosis but show less inflammation and less mucus cyst(s) but with atypical ductular structures and can metastasize. It has been proposed to diagnose this specific form of cholangiocarcinoma as ‘‘cholangiocarcinoma, intestinal type’’ (Greaves 2007), but that nomenclature as a separate (sub-) diagnosis was not favorably encouraged at a recent scientific workshop (NTP Satellite Symposium 2010).
Adenoma, Hepatocholangiocellular (Figures 149 and 150)
Pathogenesis: Proliferation of admixture of hepatocytes and intrahepatic bile duct epithelium. Stem cell origin speculated.
Diagnostic features:
- Features of hepatocellular adenoma and cholangioma are present.
- Areas composed of cords of neoplastic hepatocellular cells merge with areas composed of ducts lined by neoplastic epithelium that resembles bile duct epithelium. Hepatocytes may be seen forming ductules or ducts.
- The neoplastic biliary epithelium forms slightly dilated acini lined by cuboidal cells.
- Stratification and atypia of the biliary epithelium is minimal or absent.
- Stromal component may be absent.
- Hepatocytes may have some alteration in staining (eosinophilic, basophilic, or clear cell) compared to surrounding parenchyma.
- Mitotic figures are rare.
Differential diagnosis:
- Adenoma, hepatocellular—composed of neoplastic hepatocytes. Loss of normal lobular architecture with irregular growth pattern. Liver plates often impinge perpendicular or obliquely on the surrounding parenchyma. Distinct compression is present.
- Cholangioma—composed of neoplastic duct epithelium. Well demarcated; usually solitary. The acini are lined by cuboidal uniform and well differentiated epithelium.
- Carcinoma, hepatocholangiocellular—features of hepatocellular carcinoma and cholangiocarcinoma are present. Hepatocytes arranged in nests, solid, trabecular, or glandular patterns. Stratification and atypia of biliary epithelial component is present.
Comment: Rare spontaneous lesion. A proliferative mixture of hepatocytes and intrahepatic bile duct epithelium with neither component being malignant comprise to this tumor type (Deschl et al. 2001; Frith and Ward 1979; Harada et al. 1999; Narama et al. 2003).
Carcinoma, Hepatocholangiocellular (Figures 151 and 152)
Pathogenesis: Proliferation of admixture of hepatocytes and intrahepatic bile duct epithelium. Stem cell origin speculated.
Diagnostic features:
- Features of hepatocellular carcinoma and cholangiocarcinoma are present.
- The hepatocytic component may be arranged in trabecular, glandular, or solid patterns.
- The biliary component may form acini or small nests without lumen. Stratification or cellular atypia may occur.
- Occasionally ducts lined by both cell types, hepatocytic and biliary epithelial cells, may be present.
- Areas of hemorrhage and necrosis may be present. Mitotic figures may be numerous.
Differential diagnosis:
- Carcinoma, hepatocellular—composed of malignant hepatocytes.
- Cholangiocarcinoma—composed of malignant duct epithelium.
- Adenoma, hepatocholangiocellular—features of hepatocellular adenoma and cholangioma are present. Hepatocytic component has typical cord-like arrangement of cells. Evidence of stratification and atypia of biliary epithelial component is minimal or absent.
Comment: Rare spontaneous lesion. This neoplasm contains neoplastic elements of both hepatocytes and bile duct epithelium (Deschl et al. 2001; Frith and Ward 1979; Harada et al. 1999; Narama et al. 2003; Teredesai, Wohrmann, and Schlage 2002). A diagnosis of malignancy may be based on just one of the components being malignant.
Tumor, Ito Cell, Benign (Figures 153 and 154)
Synonyms: Fat-storing cell tumor; stellate cell tumor, lipoma.
Pathogenesis: Arises from fat-storing perisinusoidal cells, so-called Ito cells.
Diagnostic features:
- Unicentric or multicentric unencapsulated mass.
- Focal or diffuse accumulation of tumor cells.
- Partially distinct compression of adjacent hepatic parenchyma.
- May grow in sheets, clusters, or along cords of hepatocytes.
- Cells vary in size and shape and are vacuolated.
- Multiple cytoplasmic fat droplets of different size occur.
- The nuclei are ovoid or round and may be indented by cytoplasmic lipid droplets.
- Frequently evidence of atrophy of adjacent liver tissue is present.
- Modest amount of collagenous matrix may be present.
- At the margin of the lesion, spindle-shaped cells are frequently observed.
- Unencapsulated.
Differential diagnosis:
- Ito cell hyperplasia—multicentric lipomatous lesion or a singular small lesion that does not show distinct compression of surrounding liver parenchyma.
- Liposarcoma—various types of fat cells, foam cells, giant cells, myxoid cells, or fibroblast-like cells may be present.
Comment: Ito cell neoplasms are extremely rare. As a consequence, the histogenesis and the biological behavior of these tumors are not well established (Dixon et al. 1994; Enzan 1985; Tillmann et al. 1997).
Histiocytic Sarcoma (Figure 155)
Synonym: Kupffer cell sarcoma.
Pathogenesis: May arise from fixed macrophages (Kupffer cells) attached to the sinusoidal endothelial cells or from circulating macrophages, unless metastasized from other organs (e.g., skin, uterus).
Diagnostic features:
- Characteristically form nodules within the liver that may contain a central area of necrosis surrounded by palisaded tumor cells (multicentric origin).
- Uniform population of rounded or oval cells with foamy, eosinophilic cytoplasm, indistinct cell boundaries, and elongated or folded nuclei.
- Sometimes are present multinucleated giant cells of foreign body scattered throughout the tumor.
- Atypical cells are sparse, pleomorphism is usually absent, and mitotic figures may be numerous.
- The tumors grow along sinusoids and vessels and frequently involve other organs such as the lung and spleen.
- Metastases and spread on serosal surfaces and in the vascular spaces are common.
- Minimal fibrosis may be present.
Differential diagnosis:
- Malignant fibrous histiocytoma—the tumor has a mixed cell population of histiocyte-like cells, bizarre tumor giant cell, fibroblasts, and undifferentiated cells. The fibrous component is always prominent.
- Malignant lymphoma—no giant cells are seen and lymph nodes and spleen are frequently involved. Histiocytic sarcoma and lymphoma may occur together in the liver.
Comment: Histiocytic sarcomas occur at a low frequency in rats and mice (Harada et al. 1999; Eustis et al. 1990). The tumor can be part of a systemic lesion involving various tissues (spleen, lung, and uterus); when involving only the liver it is sometimes referred to as Kupffer cell sarcoma (Deschl et al. 2001; Carlton et al. 1992).
Hemangioma (Figures 156 and 157)
Synonym: Hemangioendothelioma, benign.
Pathogenesis: Arises from endothelial cells lining vascular spaces, most commonly of the hepatic sinusoids.
Diagnostic features:
- A moderate compression of the surrounding tissues is usually seen.
- The tumor is rarely encapsulated.
- Blood-filled spaces lined with a single layer of prominent uniform endothelial cells without atypia.
- Mitotic figures are rarely present.
- Solid cellular areas of uniform cells without atypia may occur.
- Often multifocal in the liver or in livers with angiectasis of mice.
Capillary type:
- Closely packed capillary structures.
- Minimal stroma between the vascular spaces.
- Cavernous type:
- Large vascular channels.
- Abundant connective tissue between the larger channels.
Differential diagnosis:
- Angiectasis—dilated vessels or sinusoids are not increased in number and have normal structure and well-differentiated endothelial cells.
- Hyperplasia, angiomatous—the hyperplastic vessels are lined by a normal endothelium and cause no or only minimal compression of the surrounding tissues.
- Lymphangioma—the vascular spaces are devoid of erythrocytes.
- Hemangiosarcoma—cytological and histological features of malignancy are present, such as cellular pleomorphism, increased mitotic activity, tissue invasion, or metastases.
- Hemangiopericytoma, benign—the tumor consists of tightly packed spindle-shaped tumor cells encircling thin-walled vascular channels (‘‘fingerprints’’). In reticulin-stained sections, a dense reticulin meshwork surrounds individual tumor cells.
Comment: The occurrence of hemangiomas in rodents has been well documented (Booth and Sundberg 1996; Carter 1973; Faccini, Abbott, and Paulus 1990; Frith and Ward 1988; Frith and Wiley 1982; Heider and Eustis 1994; Jones and Butler 1975; Maita et al. 1988; Greaves and Barsoum 1990; Greaves and Faccini 1984; Mitsumori 1990; Peckham and Heider 1999; Stewart 1979; Stewart et al. 1980; Squire and Levitt 1975; Ward et al. 1979; Yamate et al. 1988; Zwicker et al. 1995). The cavernous type of hemangioma is considered by some authors to be a congenital malformation rather than a neoplasm.
Hemangiosarcoma (Figures 158-160)
Synonym: Hemangioendothelioma, malignant.
Pathogenesis: Arises from pluripotential mesenchymal stem cells; endothelial cells of blood vessels or hepatic sinusoids.
Diagnostic features:
- The endothelial lining cells have a moderate pleomorphism.
- Endothelial cells may be multilayered and/or clustered.
- Various vascular patterns may be present, but vessels are not well formed.
- Undifferentiated or fibrosarcomatous areas may also be seen.
- Mitotic figures are often present.
- Local invasion and metastases are often present.
Differential diagnosis:
- Granulation tissue—the newly formed blood vessels are typically arranged perpendicular to fibroblasts, collagen bundles, and surfaces with no cytological and histological features of malignancy.
- Hemangioma—no cytological and histological features of malignancy such as cellular pleomorphism, increased mitotic activity, tissue invasion, or metastases are present.
- Hemangiopericytoma, malignant—the tumor consists of tightly packed spindle-shaped tumor cells that have cytological features of malignancy and encircle thin-walled vascular channels (‘‘fingerprints’’).
- Fibrosarcoma—the tumor lacks a distinct vascular pattern with prominent endothelial cells.
Comment: As in the case of hemangiomas, the occurrence of hemangiosarcomas in rodents has been well described in the published literature (Binhazim, Coghlan, and Walker 1994; Booth and Sundberg 1996; Faccini, Abbott, and Paulus 1990; Frith and Ward 1988; Frith and Wiley 1982; Giddens and Renne 1985; Greaves and Barsoum 1990; Greaves and Faccini 1984; Heider and Eustis 1994; Jones and Butler 1975; Maita et al. 1988; Mitsumori 1990; Morgan et al. 1984; Peckham and Heider 1999; Popper, Maltoni, and Selikoff 1981; Pozharisski and Turusov 1991; Sakamoto, Takayama, and Hosoda 1989; Solleveld et al. 1988; Stewart 1979; Yamate et al. 1988).
Endothelial cell-derived hemangiosarcomas can be induced in rats and mice by a wide range of industrial, natural, and pharmaceutical compounds. There are numerous examples documenting the progress that is being made in recent years in suggesting the genesis and potential relevance for human risk assessment of these tumors (Klaunig and Kamendulis 2005; Laifenfeld et al. 2010; Ohnishi et al. 2007).
H. Other Liver Lesions
Extramedullary Hematopoiesis (Figures 161 and 162)
Synonym: Hematopoietic cell proliferation; myelopoiesis; erythropoiesis.
Pathogenesis: In adult rodent, a response to increased hematopoietic demand.
Diagnostic features:
- Small aggregates of hematopoietic cells are randomly distributed in the hepatic sinusoids as well as around central veins and in portal areas.
- Both erythroid and granulocytic cells may be present in the aggregates; rarely megakaryocytes may be present.
- Typically, not associated with hepatocellular necrosis or degeneration.
Differential diagnosis:
- Mononuclear cell aggregates—lymphocytes and histiocytic cells present alone or in addition to mature myeloid cells.
- Focal inflammation—mixed mature leukocytes, often associated with or a response to cellular necrosis.
Comment: Extramedullary hematopoiesis (EMH) can be observed in rodent liver occasionally in response to an increased hematopoietic demand. Hematopoiesis is normally found in the embryonic liver where embryonic hematopoiesis dramatically expands at mid-gestation but decreases after birth. Hepatic EMH is seen more common in rodents than in man, more common in mice than rats, and more often observed in females as compared to males as a general rule (Eustis et al. 1990; Harada et al. 1996, 1999). Precipitating factors for the occurrence are: anemia, stress, xenobiotic toxicity, infection, neoplasia (e.g., histiocytic sarcoma), and pregnancy. When the erythroid precursors predominate, often the term extramedullary erythropoiesis is used.
Intrahepatocellular Erythrocytes (Figure 163)
Synonyms: Emperipolesis, cytoplasmic inclusions; hepatic erythrophagocytosis.
Pathogenesis: Unknown.
Diagnostic features:
- Individual or small clusters of enlarged hepatocytes containing intact erythrocytes.
- Affected hepatocytes are markedly enlarged.
- Marginated hepatocyte nucleus.
Differential diagnosis:
- Angiectasis—dilated vascular spaces lined by endothelial cells; dilated vascular channels and spaces frequently contain erythrocytes.
- Cystic degeneration—consists of enlarged stellate cells with flocculent eosinophilic cytoplasm.
Comment: The intracytoplasmic inclusion of large numbers of erythrocytes in hepatocytes has been seen exclusively in mice (Harada et al. 1999). It has occurred in at least nine separate cancer bioassays and one fourteen-day study in B6C3F1 mice. In two of these studies, it appears to have been exacerbated or possibly caused by treatment. Attempts to demonstrate active erythrophagocytosis by electron microscopy have been unsuccessful. A potential mechanism is emperiopolesis. Internalization of erythrocytes in hepatocytes has been reported in hibernating frogs (Barni and Bernocchi 1991).
Pancreatic Acinar Metaplasia (Figures 164 and 165)
Pathogenesis: Islands of pancreatic tissue localized within the hepatic parenchyma are rare spontaneous occurrences in rats but have been reported following prolonged exposures to polychlorinated biphenyls (Kimbrough 1973; Eustis et al. 1990; Greaves 2007).
Diagnostic features:
- The islands of pancreatic tissue resemble normal acinar pancreas with zymogen granules.
- An integrated component of the hepatic tissue.
Differential diagnosis:
- Artifact during tissue processing—tissue ‘‘floater.’’
- Metastatic pancreatic acinar neoplasia—locally invasive with tissue destruction; presence of a primary pancreatic acinar neoplasm in the pancreas.
- Ectopic pancreatic tissue—a collection of pancreatic tissue adjacent to but not integrated within hepatic parenchyma.
Comment: In spontaneous cases, a distinction between metaplasia and ectopic pancreas may not be possible. Since both pancreas and liver are embryologically related, there is a definite potential for metaplasia.
Hepatocytes, Glandular Metaplasia (Figures 166 and 167)
Pathogenesis: Proliferation of hepatocytes to form glandular structures.
Diagnostic features:
- A few to numerous glandular structures diffusely scattered in the hepatic parenchyma.
- May also be present in hyperplastic nodules and hepatocellular adenomas.
- Vary in size from one to ten times the diameter of a portal bile duct.
- Lining cells resemble hepatocytes but smaller more cuboidal glandular cells resemble biliary epithelium.
- Glandular lumen may contain granular eosinophilic material and sometime free blood cells.
Differential diagnosis:
- Cholangioma; glandular acini may vary in size and shape, expansively growing with compression.
- Hepatocholangioma; features of hepatocellular adenoma and cholangioma are present.
Comment: Partial replacement of hepatic parenchyma by glandular structures with features resembling hepatocytes has been observed in chronic studies of 3, 3’, 4, 4’, 5-pentachlorobiphenyl and 2, 3’, 4, 4’, 5-pentachlorobiphenyl (NTP Toxicology and Carcinogenesis Studies 2006). It is speculated that the glandular structures represent abnormal differentiation of hepatic precursor cells (NTP Toxicology and Carcinogenesis Studies 2006).
Intravascular Hepatocytes (Figure 168)
Synonyms: Vascular pseudoinvasion; vascular infiltration of hepatocytes.
Pathogenesis: Unknown. Sporadic occurrence.
Diagnostic features:
- Protrusion of normal appearing hepatocytes into hepatic veins and within the contour of the vessel.
- Usually involves medium to large size hepatic veins.
- Infiltrating hepatocytes are covered by an endothelial cell lining.
Differential diagnosis:
- Extension of perivascular focus of cellular alteration, usually basophilic.
- Metastatic hepatocellular carcinoma.
Comment: Intravascular infiltration of hepatocytes is rarely seen in control and treated mice. A similar change was reported in diethylnitrosamine treated mice as part of a basophilic focus of cellular alteration response (Goldfarb et al. 1983; Koen, Pugh, and Goldfarb 1983). The significance of this change is unknown.
I. Gallbladder Lesions
Congenital Lesions
Heterotopic Hepatocytes
Pathogenesis: Developmental anomaly; postnatal transdifferentiation.
Diagnostic features:
- Cells morphologically identical to mature hepatocytes are present in the submucosa of the gallbladder (Harada et al. 1999).
Differential diagnosis:
- Metastatic hepatocellular neoplasm—locally invasive expansile mass of atypical hepatocytes.
- Artifact—tissue ‘‘floater.’’
Heterotopic Acinar Pancreas (Figures 169 and 170)
Pathogenesis: Developmental abnormality; postnatal transdifferentiation.
Diagnostic features:
- Mature pancreatic acinar tissue located in wall of the gallbladder (Harada et al. 1999).
Differential diagnosis:
- Metastatic pancreatic acinar carcinoma—locally invasive neoplasm with distortion and/or destruction of gallbladder tissue.
- Pancreatic metaplasia—smoothly integrated normal appearing pancreatic tissue within the gallbladder mucosa.
Degenerative Lesions
Hyalinosis, Gallbladder (Figures 171–174)
Synonyms: Hyalinosis, cytoplasmic inclusions, crystals.
Pathogenesis: A change in the gallbladder epithelium that can be induced by inflammation and unknown factors.
Diagnostic features:
- The gallbladder epithelial cells contain a hyaline cytoplasm, which is uniformly eosinophilic.
- The protein is usually immunoreactive for Ym1/Ym2.
- Epithelial cells may contain eosinophilic needle-like crystals and the same and larger crystals may be seen extracellularly.
- Maybe associated with hyalinosis in other tissues, such as bile duct epithelium, stomach, and lung.
Differential diagnosis:
- Other degenerative cellular changes without hyalinized eosinophilic cytoplasm.
- Amyloidosis—pale eosinophilic extracellular deposits.
Comment: The hyaline protein in the cells has been shown to be Ym1/Ym2 (now Chi313), a chitinase-like protein, with unknown functions. In sickle cell mice, it is associated with gallstones. Hyalinosis is rare in most lines of mice (Harada et al. 1999; Hsu et al. 2006; Yang and Campbell 1964) but may occur in high incidence in 129 and B6;129 mice (Ward et al. 2001) and in some genetically engineered mouse lines. Hyalinosis is reported to occur in increased incidence in B6C3F1 female mice exposed to penicillin.
Glandular Metaplasia
Synonym: Adenomatoid change.
Pathogenesis: Spontaneous and associated with inflammatory and proliferative changes in gallbladder.
Diagnostic features:
- Thickened mucosa with diffuse or focal proliferation of tall columnar cells forming numerous glands in the lamina propria.
- The gallbladder epithelial cells may show increased cell proliferation.
- Hypertrophic columnar cells with uniform, homogeneous, bright eosinophilic cytoplasm forming glandular structures with lumen containing eosinophilic crystals.
- Chronic inflammation of the gallbladder may be present.
Comment: Glandular metaplasia has been observed at low prevalence in the gall bladder and intra-hepatic bile ducts (all associated with cholelithiasis), cholecystitis, cholangitis, papillomatous hyperplasia, papilloma, intra-mural cysts, and focal epithelial ulceration of aged mice from life span carcinogenicity studies. The lesions are found predominantly in female mice (Lewis 1984) and can occur spontaneously in some strains of mice. Other metaplastic changes have also been described in the human gallbladder, including goblet cells, paneth cells, and/or enterochromaffin cells in the mucosa (Hruban, Argani, and Ali 2006).
Gallbladder, Calculi (Figures 175 and 176)
Synonyms: Stones, gallstones, choleliths.
Pathogenesis: Excess dietary factors and altered metabolism.
Diagnostic features:
- Grossly visible concretion(s) in the gallbladder of mice.
- Grossly, may be solid or soft, single or multiple, and of various colors including white and pigmented (yellow, grey).
- Depending on the etiology, the stone may be composed of a mixture of cholesterol, calcium salts, hemoglobin, and occasionally as a pure stone composed of just one of these substances.
- Often associated with inflammatory lesions of the gallbladder.
Differential diagnosis:
- Mineralization—associated with necrosis, necrotic benign or malignant tumor, and inflammatory conditions.
- Neoplasia (Gross)—histologically, it is neoplastic.
- Inflammation—inflammatory exudates may be seen without gallstone formation.
- Parasites—can be seen histologically as parasites.
Comment: Gallstones are rare spontaneously in mice but may be experimentally induced by various methods (Chang, Suh, and Kwon 1999; Hsu et al. 2006; Ichikawa et al. 2009; Lee and Scott 1982; Lewis 1984; Rege and Prystowsky 1998; Tepperman, Caldwell, and Tepperman 1964; Trotman et al. 1983; Xie et al. 2009).
Inflammatory Lesions
Cholecystitis (Figures 177 and 178)
Synonym: Inflammation, gallbladder.
Pathogenesis: Toxicant exposure and bacterial and viral infections (Greaves 2007; Harada et al. 1999).
Diagnostic features:
- May be accompanied by ulcers or erosions of the mucosal lining cells.
- Types of inflammation range from acute to chronic, including a granulomatous reaction.
- Lumen may contain necrotic cellular debris.
- Mucosal hyperplasia and mucinous metaplasia may be present in some cases of inflammation.
- Initial change may be submucosal edema.
Proliferative Lesions
Hyperplasia, Gallbladder (Figures 179 and 180)
Pathogenesis: Irritation of gallbladder mucosa and after xenobiotic exposure.
Diagnostic features:
- Lesion is often small.
- Lesion varies from a few cells on papillary folds to small papillary projections.
- Epithelium is usually single layered.
- Cells are well differentiated.
- Minimal atypia may be present.
Differential diagnosis:
- Adenoma—growth pattern is disordered. May exhibit some atypia
- Adenocarcinoma—increased cellular atypia. Disordered growth pattern. Invasive growth.
- Adenomatoid change/glandular metaplasia—focal or diffuse proliferation of epithelial cells forming glandular structures. Cells are well differentiated, usually columnar, and eosinophilic, with little or no cellular atypia. Distinct eosinophilic crystals are present in cytoplasm, or in lumen of glands.
Comment: Details related to gallbladder hyperplasia can be found in several references (Deschl et al. 2001; Harada et al. 1996, 1999; Yoshitomi, Alison, and Boorman 1986; Yoshitomi and Boorman 1994).
Adenoma, Gallbladder (Figures 181–183)
Synonym: Adenoma, papillary.
Pathogenesis: Arises from epithelium of gallbladder.
Diagnostic features:
- In general, well differentiated and solitary.
- Growth is disordered, predominantly papillary- or cauliflower-like.
- Epithelium is single layered, but occasionally may be multilayered.
- Amount of fibrovascular stroma is variable.
- Cells may exhibit some atypia, with enlarged nuclei or even giant nuclei with one or two nucleoli.
- Mitotic figures may be present.
- Often inflammatory cellular infiltration and focal mineralization of the stroma may be present.
Differential diagnosis:
- Hyperplasia (focal)—small lesion. Exhibits only little evidence of atypia.
- Adenocarcinoma—increased cellular atypia. Disordered growth pattern. Invasive growth.
- Adenomatoid change/glandular metaplasia—focal or diffuse proliferation of epithelial cells forming glandular structures. Cells are well differentiated, usually columnar, and eosinophilic, with little or no cellular atypia. Distinct eosinophilic crystals are present in cytoplasm, or in lumen of glands.
Comment: Benign and malignant epithelial neoplasms of the gallbladder are described in several published references (Deschl et al. 2001; Harada et al. 1996, 1999; Lewis 1984; Yoshitomi, Alison, and Boorman 1986; Yoshitomi and Boorman 1994).
Adenocarcinoma, Gallbladder
Pathogenesis: Arises from epithelium of the gallbladder.
Diagnostic features:
- May be a sessile broad-based mass or characterized by diffusely thickening of mucosa.
- Growth pattern is disordered.
- Cellular atypia is present.
- Cytoplasm is scant and basophilic.
- Nuclei are enlarged.
- Mitotic figures are common.
- Invasion of the wall of the gallbladder or of adjacent tissue.
Differential diagnosis:
- Hyperplasia—lesion is small. Exhibits only little evidence of atypia.
- Adenoma—no evidence of invasive growth. Less cellular atypia.
- Adenomatoid change (glandular metaplasia)—focal or diffuse proliferation of epithelial cells forming glandular structures. Cells are well differentiated, usually columnar, and eosinophilic, with little or no cellular atypia. Distinct eosinophilic crystals are present in cytoplasm, or in lumen of glands.
Comment: Benign and malignant epithelial neoplasms of the gallbladder are described in several published references (Deschl et al. 2001; Harada et al. 1996, 1999; Lewis 1984; Yoshitomi, Alison, and Boorman 1986; Yoshitomi and Boorman 1994).































Acknowledgements
The authors wish to express their thanks for the excellent editorial support and suggestions provided by John Vahle, Bob Greenhill, John Foster, Chirukandath Gopinath, Rupert Kellner, Beth Mahler, Stuart Levin, Ken Schafer, Gordon Hard, Ian Pyrah, Charlotte Keenan, Maria-Luisa Phan Lung Whu, and Suzy Tirtodikromo. Special thanks to Beth Mahler and Emily Singletary for photography support. The majority of the photomicrographs used in this document were provided courtesy of the National Toxicology Program Archives, National Institute of Environmental Health Sciences, Research Triangle Park, North Carolina.
References
Aigelsreiter, A., Janig, E., Stumptner, C., Fuchsbichler, A., Zatloukal, K., and Denk, H. (2007). How a cell deals with abnormal proteins. Pathogenetic mechanisms in protein aggregation diseases. Pathobiology 74, 145–58. 10.1159/000103374
Anderson, N., and Borlak, J. (2006). Drug-induced phospholipidosis. FEBS Lett 580, 5533–540. 10.1016/j.febslet.2006.08.061
Atzori, L., and Congiu, L. (1996). Effect of verapamil on allyl alcohol hepatotoxicity. Drug Metabol Drug Interact 13, 87–98. 10.1515/dmdi.1996.13.2.87
Aydin, G., Ozçelik, N., Ciçek, E., and Soyöz, M. (2003). Histopathologic changes in liver and renal tissues induced by Ochratoxin A and melatonin in rats. Hum Exp Toxicol 22, 383–91. 10.1191/0960327103ht354oa
Babich, M. A., Chen, S. B., Greene, M. A., Kiss, C. T., Porter, W. K., Smith, T. P., Wind, M. L., and Zamula, W. W. (2004). Risk assessment of oral exposure to diisononyl phthalate from children’s products. Regul Toxicol Pharmacol 40, 151–67. 10.1016/j.yrtph.2004.06.005
Bach, U., Hailey, J. R., Hill, G. D., Kaufmann, W., Latimer, K. S., Malarkey, D. E., Maronpot, R. M., and Elmore, S. A. (2010). Proceedings of the 2009 National Toxicology Program Satellite Symposium. Toxicol Pathol 38, 9–36. 10.1177/0192623309354111
Bannasch, P. (2003). Comments on R. Karbe and R. L. Kerlin (2002). Cystic degeneration/spongiosis hepatis (Toxicol Pathol 30, 216–227). Toxicol Pathol 5, 566–70. 10.1080/019262302753559551
Bannasch, P., Bloch, M., and Zerban, H. (1981). Spongiosis hepatis. Specific changes of the perisinusoidal liver cells induced in rats by N-nitrosomorpholine. Lab Invest 44, 252–64.
Bannasch, P., Brenner, U., and Zerban, H. (1985). Cholangiofibroma and cholangiocarcinoma, liver, rat. In Monographs on Pathology of Laboratory Animals. Digestive System (T. C. Jones, U. Mohr, and R. D. Hunt, eds.), pp. 52–65. Springer, New York.
Bannasch, P., Wayss, K., and Zerban, H. (1997). Peliosis hepatis, rodents. In Digestive System (T. C. Jones, U. Mohr, and R. D. Hunt, eds.), pp. 154–60. Springer-Verlag, Berlin, New York.
Bannasch, P., and Zerban, H. (1990). Tumours of the liver. In Pathology of Tumours in Laboratory Animals. Vol. I: Tumours of the Rat (V. S. Turusov and U. Mohr, eds.), pp. 199–240, 2nd edition. IARC Scientific Publications No. 99, Lyon, France.
Bannasch, P., Zerban, H., and Hacker, H. J. (1985). Foci of altered hepatocytes, rat. In Monographs on Pathology of Laboratory Animals. Digestive System (T. C. Jones, U. Mohr, and R. D. Hunt, eds.), pp 10–30. Springer, New York.
Barni, S., and Bernocchi, G. (1991). Internalization of erythrocytes into liver parenchymal cells in naturally hibernating frogs (Rana esculenta L.). J Exp Zoology 258, 143–50. 10.1002/jez.1402580202
Belloni, A. S. Rebuffat, P., Gottardo, G., Meneghelli, V., Coi, A., Mazzocchi, G., and Nussdorfer, G. G. (1988). A morphometric study of the effects of short-term starvation on rat hepatocytes. J Submicrosc Cytol Pathol 20, 751–57.
Beregi, E., Penzes, L., Regius, O., and Izsak, J. (1987). Biological changes and diseases in aged CBA/Ca mice. Compr Gerontol [A] 1, 72–4.
Bergs, V. V., and Scotti, T. M. (1967). Virus-induced peliosis hepatitis in rats. Science 158, 377–78. 10.1126/science.158.3799.377
Binhazim, A. A., Coghlan, L. G., and Walker, C. (1994). Spontaneous hemangiosarcoma in the tail of a Long-Evans rat carrying the Eker mutation. Lab Anim Sci 44, 191–94.
Booth, C. J., and Sundberg, J. P. (1996). Hemangiomas and hemangiosarcomas. In Pathobiology of the Aging Mouse. Vol 1. Cardiovascular System (U. Mohr, D. L. Dungworth, C. C. Capen, W. W. Carlton, J.P. Sundberg, and J. M. Ward, eds.), pp 393–401. ILSI Press, Washington, DC.
Bourdeau, A., Faughnan, M. E., McDonald, M. L., Paterson, A. D., Wanless, I. R., and Letarte, M. (2001). Potential role of modifier genes influencing transforming growth factor-beta1 levels in the development of vascular defects in endoglin heterozygous mice with hereditary hemorrhagic telangiectasia. Am J Pathol 158, 2011–020. 10.1016/s0002-9440(10)64673-1
Boyd, M. R. (1981). Toxicity mediated by reactive metabolites of furans. Adv Exp Med Biol 136, B865–79.
Breitschwerdt, E. B., and Kordick, D. L. (2000). Bartonella infection in animals: Carriership, reservoir potential, pathogenicity, and zoonotic potential for human infection. Clin Microbiol Rev 13, 428–38. 10.1128/CMR.13.3.428
Brooks, P. N., and Roe, F. J. C. (1985). Hepatocellular adenoma, liver, rat. In Monographs on Pathology of Laboratory Animals. Digestive System (T. C. Jones, U. Mohr, and R. D. Hunt, eds.), pp. 47–52. Springer, New York.
Browning, F. M., Schroeder, C. R., and Berringer, O. M. (1974). An Atlas and Dissection Manual of Rhesus Monkey Anatomy. 2nd printing. Rose Printing Company Inc., Tallahassee, Florida.
Bruguera, M. (1999). Biopsia hepática en las enfermedades hepáticas por depósito. Gastroenterología Y Hepatología 22, 20–4.
Bruguera, M., Aranguibel, F., Ros, E., and Rodes, J. (1978). Incidence and clinical significance of sinusoidal dilatation in liver biopsies. Gastroenterology 75, 474–78. https://doi.org/10.1016/0016-5085(78)90853-3
Bruni, C. (1960). Hyaline degeneration of rat liver cells studied with the electron microscope. Lab Invest 9, 209–15.
BSTP. (2007). Modular Education Programme in Toxicological Pathology. Module 8: Liver. Cambridge University, Department of Pathology, Cambridge, UK.
Burek, J. D. (1978). Pathology of Aging Rats. CRC Press, West Palm Beach, Florida.
Burt, A. D., Portmann, B. C., and MacSween, R. N. M. (2002). Liver pathology associated with diseases of other organs or systems. In Pathology of the Liver (R. N. M. MacSween, ed.). 4th edition, pp. 827–83. Churchill Livingstone, London.
Carlton, W. W., Ernst, H., Faccini, J. M., Greaves, P., Krinke, G. J., Long, P. H., Maekawa, A., Newsholme, S. J., and Weisse, G. (1992). Soft tissue and musculoskeletal system 2. In International Classification of Rodent Tumours—Part 1—The Rat (U. Mohr, ed.), p. 31. WHO IARC Scientific Publications, Lyon, France.
Carter, R. L. (1973). Tumours of the soft tissues. In Pathology of Tumours in Laboratory Animals. Vol I. Tumours of the Rat, Part 1 (V. S. Turusov, ed.), pp 151–68. IARC Scientific Publications, Lyon, France.
Chang, H. J., Suh J. I., and Kwon, S. Y. (1999). Gallstone formation and gallbladder mucosal changes in mice fed a lithogenic diet. J Korean Med Sci 14, 286–92. 10.3346/jkms.1999.14.3.286
Chatman, L. A., Morton, D., Johnson, T. O., and Anway, S. D. (2009). A strategy for risk management of drug-induced phospholipidosis. Toxicol Pathol 37, 997–1005. 10.1177/0192623309352496
Cheng, K. K. (1956). Experimental studies on the mechanism of the zonal distribution of beryllium liver necrosis. J Pathol Bacteriol 71, 265–76. 10.1002/path.1700710202
Chengelis, C. P. (1988). Changes in hepatic glutathione concentrations during carbon disulfide induced hepatotoxicity in the rat. Res Commun Chem Pathol Pharmacol 61, 97–109.
Chipchase, M. D., O’Neill, M., & Melton, D. W. (2003). Liver biology and pathobiology—Characterization of premature liver polyploidy in DNA repair (Ercc1)-deficient mice. Hepatology. Official Journal of the American Association for the Study of Liver Diseases 38, 958–66. 10.1053/jhep.2003.50421
Churukian, C. J. (2002). Pigments and minerals In Theory and Practice of Histological Techniques (J. D. Bancroft and M. Gamble, eds.), pp. 243–67. Churchill Livingstone, Edinburgh, Scotland.
Clemens, M., Jost, J. O., Kautz, G., Schwering, H., Kessler, B., and Galansky, M. (1980). Das Caroli-Syndrom. Der Chirurg; Zeitschrift Fur Alle Gebiete Der Operativen Medizen 51, 219–22.
Coe, J. E., and Ross, M. J. (1990). Amyloidosis and female protein in the Syrian hamster. Concurrent regulation by sex hormones. J Exp Med 171, 1257–267. 10.1084/jem.171.4.1257
Comporti, M. (1985). Lipid peroxidation and cellular damage in toxic liver injury. Lab Inves 53, 599–623.
Copple, B. L., Banes, A., Ganey, P. E., and Roth, R. A. (2002). Endothelial cell injury and fibrin deposition in rat liver after monocrotaline exposure. Toxicol Sci 65, 309–18. 10.1093/toxsci/65.2.309
Cullen, J. M. (2005). Mechanistic classification of liver injury. Toxicol Pathol 33, 6–8. 10.1080/01926230590522428
Datta, K., Chin, A., Ahmed, T., Qing, W. G., Powell, K. L., Simhambhatla, P., MacLeod, M. C., Stoica, G., and Kehrer, J. P. (1998). Mixed effects of 2, 6-dithiopurine against cyclophosphamide mediated bladder and lung toxicity in mice. Toxicology 125, 1–11. 10.1016/s0300-483x(97)00149-2
Davenport, M., Gonde, C., Redkar, R., Koukoulis, G., Tredger, M., Mieli-Vergani, G., Portmann, B., and Howard, E. (2001). Immunohistochemistry of the liver and biliary tree in extrahepatic biliary atresia. J Ped Surg 36, 1017–025. 10.1053/jpsu.2001.24730
Denda, A., Kitayama, W., Kishida, H., Murata, N., Tsutsumi, M., Tsujiuchi, T., Nakae, D., and Konishi, Y. (2002). Development of hepatocellular adenomas and carcinomas associated with fibrosis in C57BL/6J male mice given a choline-deficient, L-amino acid-defined diet. Jpn J Cancer Res 93, 125–32. 10.1111/j.1349-7006.2002.tb01250.x
DePass, L. R., Garman, R. H., Woodside, M. D., Giddens, W. E., Maronpot, R. R., and Weil, C. S. (1986). Chronic toxicity and oncogenicity studies of ethylene glycol in rats and mice. Fundam Appl Toxicol 7, 547–65. 10.1016/0272-0590(86)90105-3
Derelanko, M. J. (2000). Acute/chronic toxicology. In Toxicologist’s Pocket Handbook, p. 13. CRC Press, Boca Raton, Florida.
Deschl, U., Cattley, R., Harada, T., Küttler, K., Hailey, J. R., Hartig, F., Leblanc, B., Marsman, D. S., and Shirai, T. (2001). Liver, gallbladder, and exocrine pancreas, In International Classification of Rodent Tumours: The Mouse (U. Mohr, ed.), pp. 59–86. Springer, New York.
Deschl, U., Ernst, H., Frantz, J. D., Goodman, D. G., Hartig, F., Konishi, Y., Küttler, K., Popp, J. A., Püschner, H., Squire, R. A., Tatematsu, M., Tuch, K., Ward, J. M., and Whiteley L. O. (1997). Digestive system. In International Classification of Rodent Tumours Part 1—The Rat (U. Mohr, ed.), pp. 65–98. WHO IARC Scientific Publications, Lyon, France.
Diwan, B. A., Ward, J. M., Ramljak, D., and Anderson, L. M. (1997). Promotion by Helicobacter hepaticus-induced hepatitis of hepatic tumors initiated by N-nitrosodimethylamine in male A/JCr mice. Toxicol Pathol 25, 597–605. 10.1177/019262339702500610
Diwan, B. A., Ward, J. M., and Rice, J. M. (1989). Promotion of malignant ‘‘embryonal’’ liver tumors by phenobarbital: Increased incidence and shortened latency of hepatoblastomas in (DBA/2 x C57BL/6)F1 mice initiated with N-nitrosodiethylamine. Carcinogenesis 10, 1345–348. 10.1093/carcin/10.7.1345
Dixon, D., Yoshitomi, K., Boorman, G. A., and Maronpot, R. R. (1994). ‘‘Lipomatous’’ lesions of unknown cellular origin in the liver of B6C3F1 mice. Vet Pathol 31, 173–82. 10.1177/030098589403100203
Dowling, R. H. (2000). Review: Pathogenesis of gallstones. Aliment Pharmacol Ther 14, Suppl 2, 39–47. 10.1046/j.1365-2036.2000.014s2039.x
Edwards, R., Colombo, T., and Greaves, P. (2002). ‘‘Have you seen this?’’ peliosis hepatis. Toxicol Pathol 30, 521–23. 10.1080/01926230290105686
Elmore, S. (2007). Apoptosis: A review of programmed cell death. Toxicol Pathol 35, 495–516. 10.1080/01926230701320337
Emerson, Q. B., Nachtnebel, K. L., Penkava, R. R., and Rothenberg, J. (1980). Oral-contraceptive-associated liver tumors. Lancet 1, 1251. 10.1016/s0140-6736(80)91708-0
Engelhardt, N. V. (1997). Oval cells in rodent liver, mouse, rat. In Digestive System (T. C. Jones, J. A. Popp, and U. Mohr, eds.), pp. 162–66. ILSI Monograph, ILSI Press, Washington, DC.
Enomoto, M., Naoe, S., Harada, M., Miyata, K., Saito, M., and Noguchi, Y. (1974). Carcinogenesis in extrahepatic bile duct and gallbladder—carcinogenic effect of N-hydroxy-2-acetamidofluorene in mice fed a ‘‘gallstone-inducing’’ diet. Jpn J Exp Med 44, 37–54.
Enzan, H. (1985). Proliferation of Ito cells (fat-storing cells) in acute carbon tetrachloride liver injury. A light and electron microscopic autoradiographic study. Acta Pathol Jpn 35, 1301–308. 10.1111/j.1440-1827.1985.tb01429.x
Eustis, S. L., Boorman, G. A., Harada, T., and Popp, J. A. (1990). Pathology of the Fischer Rat. Reference and Atlas (G. A. Boorman, S. L. Eustis, M. R. Elwell, C. A. Montgomery, Jr, and W. F. MacKenzie, eds.), pp. 71–92. Academic Press, San Diego, California.
Evans, J. G., Collins, M. A., Lake, B. G., and Butler, W. H. (1992). The histology and development of hepatic nodules and carcinoma in C3H/He and C57BL/6 mice following chronic phenobarbitone administration. Toxicol Pathol 20, 585–94. 10.1177/019262339202000405
Evans, J. G., and Lake, B. G. (1998). The digestive system II: The hepatobiliary system In Target Organ Pathology—A Basic Text (J. Turton and J. Hooson, eds.), pp. 61–97. Taylor & Francis Ltd., London.
Faa, G., Van, E. P., Roskams, T., Miyazaki, H., Serreli, S., Ambu, R., & Desmet, V. J. (1998). Expression of cytokeratin 20 in developing rat liver and in experimental models of ductular and oval cell proliferation. J Hepatol 29, 628–33. 10.1016/s0168-8278(98)80158-x
Faccini, J. M., Abbott, D. P., and Paulus, G. J. J. (1990). Mouse Histopathology. A Glossary for Use in Toxicity and Carcinogenicity Studies. Elsevier, Oxford, UK.
Feldmann, G. (1997). Liver apoptosis. J Hepatol 26 Suppl 2, 1–11. 10.1016/s0168-8278(97)80491-6
Foster, J. R. (2000). Cell death and cell proliferation in the control of normal and neoplastic tissue growth. Toxicol Pathol 28, 441–46. 10.1177/019262330002800314
Fox, J. G., Anderson, L. C., Loew, F. M., and Quimby, F. W. (2002). Laboratory Animal Science. 2nd edition. Academic Press, San Diego, California.
Frémond, B., Jouan, H., Sameh, A. H., LeGall, E., Bergerson, C., Manac’h, A., Gruel, Y., and Babut, J. M. (1987). Tumeurs du foie secondaires á l’androgénothérapie. A propos de 2 cas chez l’enfant. Chirurgie Pédiatrique 28, 97–101.
Frith, C. H., and Ward, J. M. (1979). A morphologic classification of proliferative and neoplastic hepatic lesions in mice. J Environ Pathol Toxicol 3, 329–51. 575723
Frith, C. H., and Ward, J. M. (1988). Color Atlas of Neoplastic and Non-Neoplastic Lesions in Aging Mice. Elsevier, Oxford, UK.
Frith, C. H., Ward, J. M., and Turusov, V. S. (1994). Tumours of the Liver. Pathology of Tumours in Laboratory Animals. Vol 2. Tumours of the Mouse, 2nd edition. IARC Scientific Publications, Lyon, France.
Frith, C. H., and Wiley, L. (1982). Spontaneous hepatocellular neoplasms and hepatic hemangiosarcomas in several strains of mice. Lab Anim Sci 32, 157–62.
Gad, S. C., and Rousseaux, C. G. (2002). Use and misuse of statistics as an aid in study interpretation. In Handbook of Toxicologic Pathology (W. M. Haschek, C. G. Rousseaux, and M. A. Wallig, eds.), Vol. 1, pp. 327–418. Academic Press, San Diego, California.
Gebbia, N., Leto, G., Gagliano, M., Tumminello, F. M., and Rausa, L. (1985). Lysosomal alterations in heart and liver of mice treated with doxorubicin. Cancer Chemotherapy and Pharmacology 15, 26–30. 10.1007/BF00257289
Geller, S. A., Dahll, D., and Alsabeh, R. (2008). Application of immunohistochemistry to liver and gastrointestinal neoplasms: liver, stomach, colon, and pancreas. Arch Pathol Lab Med 132, 490–99. 10.5858/2008-132-490-AOITLA
Gerlyng, P., Abyholm, A., Grotmol, T., Erikstein, B., Huitfeldt, H. S., Stokke, T., and Seglen, P. O. (1993). Binucleation and polyploidization patterns in developmental and regenerative rat liver growth. Cell Proliferation 26, 557–65. 10.1111/j.1365-2184.1993.tb00033.x
Giddens, W. E., and Renne, R. A. (1985). Haemangiosarcoma, nasal cavity, mouse. In Monographs on Pathology of Laboratory Animals. Respiratory Systems (T. C. Jones, U. Mohr, and R. D. Hunt, eds.), pp 72–4. Springer, New York.
Gkretsi, V., Mars, W. M., Bowen, W. C., Barua, L., Yang, Y., Guo, L., St-Arnaud, R., Dedhar, S., Wu, C., and Michalopoulos, G. K. (2007). Loss of integrin linked kinase from mouse hepatocytes in vitro and in vivo results in apoptosis and hepatitis. Hepatology 45, 1025–034. 10.1002/hep.21540
Goel, S. K., Lalwani, N. D., and Reddy, J. K. (1986). Peroxisome proliferation and lipid peroxidation in rat liver. Cancer Res 46, 1324–330.
Gokalp, O., Gulle, K., Sulak, O., Cicek, E., and Altuntas, I. (2003). The effects of methidathion on liver: Role of vitamins E and C. Toxicol Ind Health 19, 63–7. 10.1191/0748233703th176oa
Goldfarb, S., Pugh, T. D., Koen, H., and He, Y. Z. (1983). Preneoplastic and neoplastic progression during hepatocarcinogenesis in mice injected with diethylnitrosamine in infancy. Environ Health Perspect 50, 149–61. 10.1289/ehp.8350149
Gonzalez-Quintela, A., Mella, C., Perez, L. F., Abdulkader, I., Caparrini, A. M., and Lojo, S. (2000). Increased serum tissue polypeptide specific antigen (TPS) in alcoholics: a possible marker of alcoholic hepatitis. Alcohol Clin Exp Res 24, 1222–226.
Goodman, D. G., Maronpot, R. R., Newberne, P. M., Popp, J. A., and Squire, R. A. (1994). Proliferative and selected other lesions in the liver of rats. In Guides for Toxicologic Pathology (STP/ARP/AFIP), pp 1–24. Society of Toxologic Pathology, Washington, DC.
Goodman, Z. D., and Ishak, K. G. (2006). Hepatobiliary system and pancreas. In Surgical Pathology and Cytopathology (S. G. Silverberg, ed.), Vol. 2, 4th ed., pp. 1465–526. Elsevier, Oxford, UK.
Gopinath, C., Prentice, D. E., and Lewis, D. J. (1987). The liver. In Atlas of Experimental Toxicological Pathology (G. A. Grasham, ed.), Vol. 13, pp. 43–60. MTP Press Limited, Lancaster, England.
Goto, K., Ohashi, H., Takakura, A., and Itoh, T. (2000). Current status of Helicobacter contamination of laboratory mice, rats, gerbils, and house musk shrews in Japan. Curr Microbiol 41, 161–66. 10.1007/s002840010111
Graewin, S. J., Lee, K. H., Tran, K. Q., Goldblatt, M. I., Svatek, C. L., Nakeeb, A., and Pitt, H. A. (2004). Leptin-resistant obese mice do not form biliary crystals on a high cholesterol diet. J Surg Res 122, 145–49. 10.1016/j.jss.2004.04.012
Graham, M. J., and Lake, B. G. (2008). Induction of drug metabolism: Species differences and toxicological relevance. Toxicology 254, 184–91. 10.1016/j.tox.2008.09.002
Gray, H., Williams, P. L., and Bannister, L. H. (1995). Gray’s Anatomy: The Anatomical Basis of Medicine and Surgery (L. H. Bannister, M. M. Berry, P. Collins, M. Dyson, J. E. Dusch, and M. W. J. Ferguson, eds.), pp 1795–802. Churchill Livingstone, New York.
Greaves, P. (2007). Liver and pancreas. In Histopathology of Preclinical Toxicity Studies. 3rd edition. pp 457–514. Elsevier, Oxford, UK.
Greaves, P., and Barsoum, N. (1990). Tumours of soft tissues. In Pathology of Tumours in Laboratory Animals. Vol. I. Tumours of the Rat, 2nd edition (V. S. Turusov and U. Mohr, eds), pp 597–23. IARC Scientific Publications, Lyon, France.
Greaves, P., Edwards, R., Cohen, G. M., and MacFarlane, M. (2001). ‘‘Have you seen this?’’ Diffuse hepatic apoptosis. Toxicol Pathol 29, 398–400. 10.1080/019262301316905363
Greaves, P., and Faccini, J. M. (1984). Rat Histopathology. A Glossary for Use in Toxicity and Carcinogenicity Studies, pp 88–97. Elsevier, Oxford, UK.
Greaves, P., and Faccini, J. M. (1992). Digestive system. In Rat Histopathology: A Glossary for Use in Toxicity and Carcinogenicity Studies, pp.105–48. Elsevier, Oxford, UK.
Greaves, P., Irisarri, E., and Monro A. M. (1986). Hepatic foci of cellular and enzymatic alteration and nodules in rats treated with clofibrate or diethylnitrosamine followed by phenobarbital: Their rate of onset and their reversibility. J Natl Cancer Inst 76, 475–84. https://doi.org/10.1093/jnci/76.3.475
Gruttadauria, S., Mandala, L., Miraglia, R., Caruso, S., Minervini, M. I., Biondo, D., Volpes, R., Vizzini, G., Marsh, J. W., Luca, A., Marcos, A., and Gridelli, B. (2007). Successful treatment of small-for-size syndrome in adult-to-adult living-related liver transplantation: single center series. Clin Transplant 21, 761–66. 10.1111/j.1399-0012.2007.00735.x
Guzman, R. E., and Solter, P. F. (2002). Characterization of sublethal microcystin-LR exposure in mice. Vet Pathol 39, 17–26. 10.1354/vp.39-1-17
Hailey, J. R., Haseman, J. K., Bucher, J. R., Radovsky, A. E., Malarkey, D. E., Miller R. T., Nyska, A., and Maronpot, R. R. (1998). Impact of Helicobacter hepaticus infection in B6C3F1 mice from twelve National Toxicology Program two-year carcinogenesis studies. Toxicol Pathol 26, 602–11. 10.1177/019262339802600503
Hailey, J. R., Walker, N. J., Sells, D. M., Brix, A. E., Jokinen, M. P., and Nyska, A. (2005). Classification of proliferative Hepatocellular lesions in Harlan Sprague-Dawley rats chronically exposed to dioxin-like compounds. Toxicol Pathol 33, 165–74. 10.1080/01926230590888324
Halliwell, W. H. (1997). Cationic amphiphilic drug-induced phospholipidosis. Toxicol Pathol 25, 53–60. 10.1177/019262339702500111
Handley, D. A., Chien, S., and Arbeeny, C. M. (1983). Ultrastructure of hepatic cholesterol crystals in the hypercholesterolemic-diabetic rat. Pathol Res Pract 177, 13–21. 10.1016/S0344-0338(83)80039-9
Hanigan, M. H., Winkler, M. L., and Drinkwater, N. R. (1993). Induction of three histochemically distinct populations of hepatic foci in C57BL/6J mice. Carcinogenesis 14, 1035–040. 10.1093/carcin/14.5.1035
Harada, T., Maronpot, R. R., Enomoto, A., Tamano, S., and Ward, J. M. (1996). Changes in the liver and gallbladder. In Pathobiology of the Aging Mouse. Vol. 2 (U. Mohr, D. L. Dungworth, C. C. Capen, W. W. Carlton, J. P. Sundberg, and J. M. Ward, eds.), pp 207–41. ILSI Press, Washington, DC.
Harada, T., Maronpot, R. R., Morris, R. W., and Boorman, G. A. (1989). Observation on altered hepatocellular foci in National Toxicology Program 2-year carcinogenicity studies in rats. Toxicol Pathol 17, 690–708. https://doi.org/10.1177/0192623389017004114
Harada, T., Maronpot, R. R., Morris, R. W., Stitzel, K. A., and Boorman, G. A. (1989). Morphological and sterological characterization of hepatic foci of cellular alteration in control Fischer 344 rats. Toxicol Pathol 17, 579–93. 10.1177/0192623389017004104
Harada, T., Enomoto, A., Boorman, G. A., and Maronpot, R. R. (1999). Liver and gallbladder, In Pathology of the Mouse (R. R. Maronpot, ed.), pp. 119–83. Cache River Press, Vienna, IL.
Harbord, M., Novelli, M., Canas, B., Power, D., Davis, C., Godovac- Zimmermann, J., Roes, J., and Segal, A.W. (2002). Ym1 is a neutrophil granule protein that crystallizes in p47phox-deficient mice. The J Biol Chem 277, 5468–475. 10.1074/jbc.M110635200
Harbrecht, B. G., Stadler, J., Demetris, A. J., Simmons, R. L., and Billiar, T. R. (1994). Nitric oxide and prostaglandins interact to prevent hepatic damage during murine endotoxemia. Am J Physiol 266, G1004–010. 10.1152/ajpgi.1994.266.6.G1004
Hardisty, J. F., Elwell, M. R., Ernst, H., Greaves, P., Kolenda-Roberts, H., Malarkey, D. E., Mann, P. C., and Tellier, P. A. (2007). Histopathology of hemangiosarcomas in mice and hamsters and liposarcomas/fibrosarcomas in rats associated with PPAR agonists. Toxicol Pathol 35, 928–41. 10.1080/01926230701748156
Hardisty, J. F., and Eustis, S. L. (1990). Toxicologic pathology: A critical stage in study interpretation. In Progress in Predictive Toxicology (D. B. Clay- son, ed.). CRC Press, Boca Raton, Florida.
Helyer, B. J., and Petrelli, M. (1978). Cytoplasmic inclusions in spontaneous hepatomas of CBA/H-T6T6 mice. Histochemistry and electron microscopy. Journal of the National Cancer Institute 60, 861–69. 10.1093/jnci/60.4.861
Heider, K., and Eustis, S. L. (1994). Tumours of the soft tissues. In Pathology of Tumours in Laboratory Animals. Vol. 2. Tumours of the Mouse, 2nd edition (V. Turusov and U. Mohr, eds.), pp. 611–49. IARC Scientific Publications, Lyon, France.
Homberger, F. R. (1996). Mouse hepatitis virus. Schweiz Arch Tierheilkd 138, 183–88.
Herman, J. R., Dethloff, L. A., McGuire, E. J., Parker, R. F., Walsh, K. M., Gough, A. W., Masuda, H., and de la Iglesia, F. A. (2002). Rodent carcinogenicity with the thiazolidinedione antidiabetic agent troglitazone. Toxicol Sci 68, 226–36. 10.1093/toxsci/68.1.226
Hruban, R. H., Argani, P., and Ali, S. Z. (2006). The pancreas and extrahepatic biliary system. In Silverberg’s Principles and Practice of Surgical Pathology and Cytopathology (G. G. Silverberg, ed.), pp. 1576–593. Churcill Livingstone, Oxford, UK.
Hruban, Z., Slesers, A., and Hopkins, E. (1972). Drug-induced and naturally occurring myeloid bodies. Lab Invest 27, 62–70.
Hsu, L., Diwan, B., Ward, J. M., and Noguchi, C. T. (2006). Pathology of ‘‘Berkeley’’ sickle-cell mice includes gallstones and priapism. Blood 107, 3414–415. 10.1182/blood-2005-11-4500
Huang, J., Ren, R. N., Chen, X. M., and Ye, L. Y. (2007). An experimental study on hepatotoxicity of topiramate in young rats. Zhongguo Dang Dai Er Ke Za Zhi 9, 54–8. http://www.zgddek.com/EN/Y2007/V9/I1/54
Hurlimann, J., and Gardiol, D. (1991). Immunohistochemistry in the differential diagnosis of liver carcinomas. Am J Surg Pathol 15, 280–88. 10.1097/00000478-199103000-00008
Hurst, E. W., and Paget, G. E. (1963). Protoporphyrin, cirrhosis and hepatoma in livers of mice given griseofulvin. Br J Dermatol 75, 105–12. https://doi.org/10.1111/j.1365-2133.1963.tb13946.x
Husztik, E., Lazar, G., and Szabo, E. (1984). Immunologically induced peliosis hepatis in rats. Br J Exp Pathol 65, 313–18. PMC2040977
Ichikawa, H., Imano, M., Takeyama, Y., Shiozaki, H., and Ohyanagi, H. (2009). Involvement of osteopontin as a core protein in cholesterol gallstone formation. J Hepatobiliary Pancreat Surg 16, 197–203. 10.1007/s00534-009-0043-4
Institute of Laboratory Animal Resources, National Research Council, National Academy of Sciences. (1980). Histologic typing of liver tumors of the rat. J Natl Cancer Inst 64, 177–206. https://doi.org/10.1093/jnci/64.1.177
Irisarri, E., and Hollander, C. F. (1994). Digestive system—Aging of the liver In Pathobiology of the Aging Rat—Vol. 2. (U. Mohr, D. L. Dungworth, and C. C. Capen, eds.), pp. 341–49. ILSI Press, Washington, DC.
Jang, J. J., Weghorst, C. M., Henneman, J. R., Devor, D. E., and Ward, J. M. (1992). Progressive atypia in spontaneous and N-nitrosodiethylamine-induced hepatocellular adenomas of C3H/HeNCr mice. Carcinogenesis 13, 1541–547. 10.1093/carcin/13.9.1541
Jones, G., and Butler, W. H. (1975). Morphology and spontaneous neoplasia. In Mouse Hepatic Neoplasia (W. M. Butler and P. M. Newberne, eds.), pp 21–59. Elsevier, Oxford, UK.
Jones, G., and Butler, W. H. (1978). Light microscopy of rat hepatic neoplasia. In Rat Hepatic Neoplasia (P. M. Newberne and W. H. Butler, eds.), pp 114–35. MIT Press, Cambridge, Massachusetts.
Jones, M. L. (2002). Lipids In Theory and Practice of Histological Techniques (J. D. Bancroft and M. Gamble, eds.), pp. 201–30. Churchill Livingstone, Edinburgh, Scotland.
Kakizoe, S., Goldfarb, S., and Pugh, T. D. (1989). Focal impairment of growth in hepatocellular neoplasms of C57BL/6 mice: A possible explanation for the strain’s resistance to hepatocarcinogenesis. Cancer Res 49, 3985–989. 2736537
Kanel, G. C., and Korula, J. (2005). Atlas of Liver Pathology, 2nd edition. Elservier Saunders, Los Angeles, California.
Karbe, E., and Kerlin, R. L. (2002). Cystic degeneration/Spongiosis hepatis in rats. Toxicol Pathol 30, 216–27. 10.1080/019262302753559551
Kari, F., Bucher, J., Haseman, J., Eusti, S., and Huff, J. (1995). Long-term exposure to the anti-inflammatory agent phenylbutazone induces kidney tumors in rats and liver tumors in mice. Jpn J of Cancer Res 86, 252–63. 10.1111/j.1349-7006.1995.tb03048.x
Kashiwagi, R., Kaidoh, T., and Inoué, T. (2001). Immunohistochemical study of dendritic cells and kupffer cells in griseofulvin-induced protoporphyric mice. Yonago Act Med 44, 7–16. https://repository.lib.tottori-u.ac.jp/817
Katoh, M., and Sugimoto, T. (1982). Effect of malotilate (diisopropyl 1, 3-dithiol-2-ylidenemalonate) on chronic liver injury caused by carbon tetrachloride. Nippon Yakurigaku Zasshi 80, 83–91.
Kiernan, F. (1883). The anatomy and physiology of the liver. Philosophical Transactions of the Royal Society of London Series B, Biol Sci 123, 711–70.
Kim, J. C., and Kaminsky, L. S. (1988). 2,2,2-Trifluoroethanol toxicity in aged rats. Toxicol Pathol 16, 35–45. 10.1177/019262338801600105
Kim, J. C., Shin, D. H., Kim, S. H., Kim, J. K., Park, S. C., Son, W. C., Lee, H. S., Suh, J. E., Kim, C. Y., Ha, C. S., and Chung, M. K. (2004). Subacute toxicity evaluation of a new camptothecin anticancer agent CKD-602 administered by intravenous injection to rats. Regul Toxicol Pharmacol 40, 356–69. 10.1016/j.yrtph.2004.09.002
Kimbrough, R. D. (1973). Pancreatic-type tissue in livers of rats fed polychlorinated biphenyls. J Natl Cancer Inst 51, 679–81. 4203081
Kimbrough, R. D., and Linder, R. E. (1974). Induction of adenofibrosis and hepatomas of the liver in BALB-cJ mice by polychlorinated biphenyls (Aroclor 1254). J Natl Cancer Inst 53, 547–52. 10.1093/jnci/53.2.547
Kimbrough, R. D., Linder, R. E., Burse, V. W., and Jennings, R. W. (1973). Adenofibrosis in the rat liver, with persistence of polychlorinated biphenyls in adipose tissue. Arch Environ Health 27, 390–95. 10.1080/00039896.1973.10666410
Kimura, M., and Abe, M. (1994). Histology of postmortem changes in rat livers to ascertain hour of death. Int J Tissue React 16, 139–50.
Kitamura, K., Iwasaki, H. O., Yasoshima, A., Yoshikawa, H., Yoshikawa, T., and Okaniwa, A. (1992). Pathology of chemically induced chronic active hepatitis in mice. Exp Mol Pathol 57, 153–66. 10.1016/0014-4800(92)90007-x
Klaunig, J. E., and Kamendulis, L. M. (2005). Mode of action of butoxyethanol-induced mouse liver hemangiosarcomas and hepatocellular carcinomas. Toxicol Lett 156, 107–15. 10.1016/j.toxlet.2003.08.012
Knasmuller, S., Parzefall, W., Helma, C., Kassie, F., Ecker, S., and Schulte-Hermann, R. (1997). Toxic effects of griseofulvin: Disease models, mechanisms, and risk assessment. Crit Rev Toxicol 27, 495–537. 10.3109/10408449709078444
Koen, H., Pugh, T. D., and Goldfarb, S. (1983). Centrilobular distribution of diethylnitrosamine-induced hepatocellular foci in the mouse. Lab Invest 49, 78–81.
König, H. E., Sautet, J., and Liebich, H. G. (2004). 7. Digestive system (apparatus digetorius). In Veterinary Anatomy of Domestic Mammals: Text-book and Colour Atlas (H. E. König, H.-G. Liebich, and H. Bragulla, eds.), pp. 332–37. Schattauer, Stuttgart.
Kuriki, J., Murakami, H., Kakumu, S., Sakamoto, N., Yokochi, T., Nakashima, I., and Kato, N. (1983). Experimental autoimmune hepatitis in mice after immunization with syngeneic liver proteins together with the polysaccharide of Klebsiella pneumoniae. Gastroenterology 84, 596–603. https://doi.org/10.1016/0016-5085(83)90085-9
Laifenfeld, D., Gilchrist, A., Drubin, D., Jorge, M., Eddy, S. F., Frushour, B. P., Ladd, B. Obert, L. A., Gosink, M. M., Cook, J. C., Criswell, K., Somps, C. J., Koza-Taylor, P., Elliston, K. L., and Lawton, M. P. (2010). The role of Hypoxia in 2-butoxyethanol–induced hemangiosarcoma. Toxicol Sci 113, 254–66. 10.1093/toxsci/kfp213
Lailach, J. J., Johnson, W. D., Raczniak, T. J., and Shumaker, R. C. (1977). Fibrin thrombosis in monocrotaline pyrrole-induced cor pulminale in rats. Arch Pathol Lab Med 101, 69–73.
Lalwani, N. D., Dethloff, L. A., Haskins, J. R., Robertson, D. G., and de La, Iglesia. (1997). Increased nuclear ploidy, not cell proliferation, is sustained in the peroxisome proliferator-treated rat liver. Toxicol Pathol 25, 165–76. 10.1177/019262339702500206
Lee, K. P. (1983). Peliosis hepatis-like lesion in aging rats. Vet Pathol 20, 410–23. 10.1177/030098588302000404
Lee, S. P., and Scott, A. J. (1982). The evolution of morphologic changes in the gallbladder before stone formation in mice fed a cholesterol-cholic acid diet. Am J Pathol 108, 1–8. PMC1916021
Levin, S. (1999). Commentary: Implementation of the STP recommendations on the nomenclature of cell death. Society of Toxicologic Pathologists. Toxicol Pathol 27, 491. 10.1177/019262339902700420
Levin, S., Bucci, T. J., Cohen, S. M., Fix, A. S., Hardisty, J. F., LeGrand, E. K., Maronpot, R. R., and Trump, B. F. (1999). The nomenclature of cell death: Recommendations of an ad hoc Committee of the Society of Toxicologic Pathologists. Toxicol Pathol 27, 484–90. 10.1177/019262339902700419
Levine, S., and Sowinski, R. (1985). Splenic infarcts produced in rats by vasoconstrictor drugs. Exp Pathol 28, 13–9. 10.1016/s0232-1513(85)80027-x
Lewis, D. J. (1984). Spontaneous lesions of the mouse biliary tract. J Comp Pathol 94, 263–71. 10.1016/0021-9975(84)90045-8
Li, X., Elwell, M. R., Ryan, A. M., and Ochoa, R. (2003). Morphogenesis of post mortem hepatocyte vacuolation and liver weight increases in Sprague-Dawley rats. Toxicol Pathol 31, 682–88. 10.1080/01926230390241981
Lin, C. C., Chang, C. H., Yang, J. J., Namba, T., and Hattori, M. (1996). Hepatoprotective effects of emodin from Ventilago leiocarpa. J Ethnopharmacol 52, 107–11. 10.1016/0378-8741(96)01397-9
Lipman, R. D., Gaillard, E. T., Harrison, D. E., and Bronson, R. T. (1993). Husbandry factors and the prevalence of age-related amyloidosis in mice. Lab Anim Sci 43, 439–44.
Lipsky, M. M., Tanner, D. C., Hinton, D. E., Trump, B. F. (1984). Reversibility, persistence, and progression of safrole-induced mouse liver lesions following cessation of exposure. In Mouse Liver Neoplasia (J. A. Popp, ed.), pp 161–77. Hemisphere Publishing Corporation, Washington, DC.
Liu, Y., Cui, D., Hoshii, Y., Kawano, H., Une, Y., Gondo, T., and Ishihara, T. (2007). Induction of murine AA amyloidosis by various homogeneous amyloid fibrils and amyloid-like synthetic peptides. Scand J Immunol 66, 495–500. 10.1111/j.1365-3083.2007.02005.x
Mahon, D. C. (1989). Altered hepatic foci in rat liver as weight of evidence of carcinogenicity: The Canadian perspective. Toxicol Pathol 17, 709–15. 10.1177/0192623389017004115
Maita, K., Hirano, M., Harada, T., Mitsumori, K., Yoshida, A., Takahashi, K., Nakashima, N., Kitazawa, T., Enomoto, A., Inui, K., and Shirasu,Y. (1988). Mortality, major cause of moribundity, and spontaneous tumors in CD-1 mice. Toxicol Pathol 16, 340–49. 10.1177/019262338801600305
Malarkey, D. E., Devereux, T. R., Dinse, G. E., Mann, P. C., and Maronpot, R. R. (1995). Hepatocarcinogenicity of chlordane in B6C3F1 and B6D2F1 male mice: Evidence for regression in B6C3F1 mice and carcinogenesis independent of ras proto-oncogene activation. Carcinogenesis 16, 2617–625. 10.1093/carcin/16.11.2617
Malarkey, D., Johnson, K., Ryan, L., Boorman, G., and Maronpot, R. (2005). New insights into functional aspects of liver morphology. Toxicol Pathol 33, 27–34. 10.1080/01926230590881826
Malhotra, V., Sakhuja, P., and Gondal, R. (2004). Immunohistochemistry in liver diseases. J Gastroenterol Hepatol 19, S364–68. https://doi.org/10.1111/j.1440-1746.2004.03704.x
Maronpot, R. R. (2009). Biological basis of differential susceptibility to hepatocarcinogenesis among mouse strains. J Toxicol Pathol 22, 11–33. 10.1293/tox.22.11
Maronpot, R. R., Harada, T., Murthy, A. S. K., and Boorman, G. A. (1989). Documenting foci of hepatocellular alteration in two-year carcinogenicity studies: Current practices of the national toxicology program. Toxicol Pathol 17, 675–84. 10.1177/0192623389017004112
Maronpot, R. R., Montgomery, C. A., Boorman G. A., and McConnell, E. E. (1986). National Toxicology Program nomenclature for hepatoproliferative lesions of rats. Toxicol Pathol 14, 263–73. 10.1177/019262338601400217
Marsman, D. S. (1995). NTP Technical Report on Toxicity Studies of Dibutyl Phthalate (CAS No. 84-74-2) Administered in Feed to F344/N Rats and B6C3F? Mice. Toxicity Report Series, No. 30. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, Research Triangle Park, North Carolina.
Marsman, D. S., and Popp, J. A. (1994). Biological potential of basophilic hepatocellular foci and hepatic adenoma induced by the peroxisome proliferator, Wy-14,643. Carcinogenesis 15, 111–17. 10.1093/carcin/15.1.111
Martignoni, M., Groothuis, G. M., and de Kanter, R. (2006). Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol 2, 875–94. 10.1517/17425255.2.6.875
Martin, N. C., McGregor, A. H., Sansom, N., Gould, S., and Harrison, D. J. (2001). Phenobarbitone-induced ploidy changes in liver occur independently of p53. Toxicology Letters 119, 109–15. 10.1016/s0378-4274(00)00299-x
Masson, R., and Roome, N. O. (1997). Spontaneous iron overload in Sprague-Dawley rats. Toxicol Pathol 25, 308–16. 10.1177/019262339702500308
Masyuk, T. V., Huang, B. Q., Masyuk, A. I., Ritman, E. L., Torres, V. E., Wang, X., Harris, P. C., and La Russo, N. F. (2004). Biliary Dysgenesis in the PCK Rat, an Orthologous Model of Autosomal Recessive Polycystic Kidney Disease. The Am J of Pathol 165, 1719–730. 10.1016/S0002-9440(10)63427-X
Masyuk, T., Masyuk, A., Torres, V., Harris, P., and Larusso, N. (2007). Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3’,5’-cyclic monophosphate. Gastroenterology 132, 1104–116. 10.1053/j.gastro.2006.12.039
Matsumoto, M., Aiso, S., Umeda, Y., Arito, H., Nagano, K., Yamamoto, S., and Matsushima, T. (2006). Thirteen-week oral toxicity of para- and ortho- chloronitrobenzene in rats and mice. J Toxicol Sci 31, 9–22. 10.2131/jts.31.9
McCaughan, G. W., Bilous, M. J., and Gallagher, N. D. (1985). Long-term survival with tumor regression and androgen-induced liver tumors. Cancer 56, 2622–626. 10.1002/1097-0142(19851201)56:11<2622::aid-cncr2820561115>3.0.co;2-0
Meierhenry, E. F., Ruebner, B. H., Gershwin, M. E., Hsieh, L. S., and French, S. W. (1983). Dieldrin-induced mallory bodies in hepatic tumors of mice of different strains. Hepatology 3, 90–5. 10.1002/hep.1840030115
Mendenhall, C. L., and Chedid, A. (1980). Peliosis hepatis. Its relationship to chronic alcoholism, aflatoxin B1, and carcinogenesis in male Holtzman rats. Digestive Diseases and Sciences 25, 587–92. 10.1007/BF01318871
Mitsumori, K. (1990). Blood and lymphatic vessels. In Pathology of the Fischer Rat. Reference and Atlas (G. A. Boorman, S. L. Eustis, M. R. Elwell, C. A. Montgomery, Jr., and W. F. MacKenzie, eds.), pp. 473–84. Academic Press, San Diego, California.
Miyai, K. (1991). The discovery of new diseases by utilizing laboratory data for clinical research. Perspectives in Biology and Medicine 34, 542–48. 10.1353/pbm.1991.0030
Miyazaki, M., Makowka, L., Falk, R. F., Falk, J. A., McDonell, M., and Venturi, D. (1983). Protection of thermochemotherapeutic-induced lethal acute hepatic necrosis in the rat by 16,16-dimethyl prostaglandin E2. J Surg Res 34, 415–26. 10.1016/0022-4804(83)90090-2
Moore, M. A., Thamavit, W., and Bannasch, P. (1996). Tumours of the liver. In Pathology of Tumours in Laboratory Animals—Vol. 3 (V. Turusov and U. Mohr, eds.), pp. 79–125. IARC, Lyon, France.
Morgan, K. T., Frith, C. H., Swenberg, J. A., McGrath J. T., Zulch K. J., and Crowder D. M. (1984). A morphologic classification of brain tumors found in several strains of mice. J Natl Cancer Inst 72, 151–60. 10.1093/jnci/72.1.151
Muff, M. A., Masyuk, T. V., Stroope, A. J., Huang, B. Q., Splinter, P. L., Lee, S. O., and Larusso, N. F. (2006). Development and characterization of a cholangiocyte cell line from the PCK rat, an animal model of Autosomal Recessive Polycystic Kidney Disease. Lab Invest 86, 940–50. 10.1038/labinvest.3700448
Naeim, F., Copper, P. H., and Semion, A. A. (1973). Peliosis hepatis. Possible etiologic role of anabolic steroids. Arch Pathol 95, 284–85. 4348726
Nagano, K., Sasaki, T., Umeda, Y., Nishizawa, T., Ikawa, N., Ohbayashi, H., Arito, H., Yamamoto, S., and Fukushima, S. (2007). Inhalation carcinogenicity and chronic toxicity of carbon tetrachloride in rats and mice. Inhal Toxicol 19, 1089–103. 10.1080/08958370701628770
Narama, I., Imaida, K., Iwata, H., Nakae, D., Nishikawa, A., and Harada, T. (2003). A review of nomenclature and diagnostic criteria for proliferative lesions in the liver of rats by a working group of the Japanese Society of Toxicologic Pathology. J Toxicol Pathol 16, 1–17. https://doi.org/10.1293/tox.16.1
Newton, P. E., Wooding, W. L., Bolte, H. F., Derelanko, M. J., Hardisty, J. F., and Rinehart, W. E. (2001). A chronic inhalation toxicity/oncogenicity study of methylethylketoxime in rats and mice. Inhal Toxicol 13, 1093–116. 10.1080/08958370152647636
Nishimura, J., Dewa, Y., Muguruma, M., Kuroiwa, Y., Yasuno, H., Shima, T., Jin, M., Takahashi, M., Umemura, T., and Mitsumori, K. (2007). Effect of fenofibrate on oxidative DNA damage and on gene expression related to cell proliferation and apoptosis in rats. Toxicol Sci 97, 44–54. 10.1093/toxsci/kfm011
Nonomura, A., Kono, N., Yoshida, K., Mizukami, Y., Nakanuma, Y., and Matsubara, F. (1991). Histological changes of the liver in experimental graft-versus-host disease across minor histocompatibility barriers. V. A light and electron microscopic study of the intralobular changes. Liver 11, 149–57. 10.1111/j.1600-0676.1991.tb00508.x
Nonoyama, T., Fullerton, F., Reznik, G., Bucci, T. J., and Ward, J. M. (1988). Mouse hepatoblastomas: A histologic, ultrastructural, and immunohistochemical study. Vet Pathol 25, 286–96. 10.1177/030098588802500407
Nonoyama, T., Reznik, G., Bucci, T. J., and Fullerton, F. (1986). Hepatoblastoma with squamous differentiation in a B6C3F1 mouse. Vet Pathol 23, 619–22. 10.1177/030098588602300511
NTP Satellite Symposium. (2010). Pathology Potpourri. Chicago Marriott Downtown, Chicago, Illinois.
NTP technical report on the toxicology and carcinogenesis studies of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin (TCDD) (CAS No. 1746-01-6) in female Harlan Sprague-Dawley rats (Gavage Studies) (2006). Natl Toxicol Program Tech Rep Ser 521, 4–232. 16835633
NTP technical report on the toxicology and carcinogenesis studies of a binary mixture of 3,3’,4,4’,5-pentachlorobiphenyl (PCB 126) (CAS no. 57465-28-8) and 2,3’,4,4’,5-pentachlorobiphenyl (PCB 118) (CAS no. 31508-00-6) in female Harlan Sprague-Dawley rats (Gavage studies). (2006). NIH Publication, No. 07-4467, Natl Toxicol Program Tech Rep Ser 531. 17342196
NTP toxicology and carcinogenesis studies of ethylene glycol (CAS No. 107-21-1) in B6C3F1 mice (feed studies). (1993). NIH Publication, No. 93-3144. Natl Toxicol. Program Tech Rep Ser 413. 12616285
NTP toxicology and carcinogenesis studies of 3, 3’, 4, 4’,5-pentachlorobiphenyl (PCB 126) (CAS No. 57465-28-8) in female Harlan Sprague-Dawley rats (Gavage Studies). (2006). Natl Toxicol Program Tech Rep Ser 520, 4-246. 16628245
NTP toxicology and carcinogenesis studies of theophylline (CAS No. 58-55-9) in F344/N Rats and B6C3F1 mice (Feed and Gavage Studies) (1998). NIH Publication, No. 98-3963, Natl Toxicol Program Tech Rep Ser 473. 12571677
Numan, F., Cokyuksel, O., Camuscu, S., Demir, K., and Duren, M. (1986). Caroli syndrome. Rontgenblatter 39, 191–92. 3529344
Nyska, A., Moomaw, C. R., Foley, J. F., Maronpot, R. R., Malarkey, D. E., Cummings, C. A., Peddada, S., Moyer, C. F., Allen, D. G., Travlos, G., and Chan, P. C. (2002). The hepatic endothelial carcinogen riddelliine induces endothelial apoptosis, mitosis, S phase, and p53 and hepatocytic vascular endothelial growth factor expression after short-term exposure. Toxicol Appl Pharmacol 184, 153–64. 10.1006/taap.2002.9485
Nyska, A., Waner, T., Wormser, U., Gur, E., Kuttin, E., and Dayan, D. (1992). Possible pitfalls in rat extended dermal toxicity testing: an hepatic-ocular syndrome. Arch Toxicol 66, 339–46. 10.1007/BF01973629
Obert, L. A., Sobocinski, G. P., Bobrowski, W. F., Metz, A. L., Rolsma, M. D., Altrogge, D. M., and Dunstan, R. W. (2007). An immunohistochemical approach to differentiate hepatic lipidosis from hepatic phospholipidosis in rats. Toxicol Pathol 35, 728–34. 10.1080/01926230701481956
Ohnishi, T., Arnold, L. L., Clark, N. M., Wisecarver, J. L., and Cohen, S. M. (2007). Comparison of endothelial cell proliferation in normal liver and adipose tissue in B6C3F1 mice, F344 rats, and humans. Toxicol Pathol 35, 904–09. 10.1080/01926230701748081
Omar, A., Elmesallamy, G.-S., and Eassa, S. (2005). Comparative study of the hepatotoxic, genotoxic and carcinogenic effects of praziquantel distocide & the natural myrrh extract Mirazid on adult male albino rats. J Egypt Soc Parasitol 35, 313–29. 15881015
Parker, G. A., and Gibson, W. B. (1995). Liver lesions in rats associated with wrapping of the torso. Toxicol Pathol 23, 507–12. 10.1177/019262339502300407
Peckham, J. C., and Heider, K. (1999). Skin and subcutis. In Pathology of the Mouse. Reference and Atlas (R. R. Maronpot, G. A. Boorman, and B. W. Gaul, eds.), pp. 555–12. Cache River Press, Vienna.
Peichoto, M. E., Teibler, P., Ruiz, R., Leiva, L., and Acosta, O. (2006). Systemic pathological alterations caused by Philodryas patagoniensis colubrid snake venom in rats. Toxicon 48, 520–28. 10.1016/j.toxicon.2006.06.013
Peraino, C., Staffeldt, E. F., Carnes, B. A., Ludeman, V. A., Blomquist, J. A., and Vesselinovitch, S. D. (1984). Characterization of histochemically detectable altered hepatocyte foci and their relationship to hepatic tumorigenesis in rats treated once with diethylnitrosamine or benzo(a)pyrene within one day after birth. Cancer Res 44, 3340–347. 6331643
Percy, D. H., and Barthold, S. W. (2001). Hamster. In Pathology of Laboratory Rodents and Rabbits, pp. 194–95. Blackwell Publishing, Ames, Iowa.
Percy, D. H., and Barthold, S. W. (2007). Pathology of Laboratory Rodents and Rabbits, 3rd edition, p. 356. Blackwell Publishing, Ames, Iowa.
Peters, M., Liebman, H. A., Tong, M. J., and Tinberg, H. M. (1983). Alcoholic hepatitis: Granulocyte chemotactic factor from Mallory body-stimulated human peripheral blood mononuclear cells. Clin Immunol Immunopathol 28, 418–30. 10.1016/0090-1229(83)90109-5
Pitt, C. G., Gupta, G., Estes, W. E., Rosenkrantz, H., Metterville, J. J., Crumbliss, A. L., Palmer, R. A., and Sciortino, C. V. (1979). The selection and evaluation of new chelating agents for the treatment of iron overload. J Pharmacol Exp Therap 208, 12–18. 759605
Popp, J. A. (1984). Mouse Liver Neoplasia. Current Perspectives. Hemisphere Publishing Co., New York.
Popp, J. A. (1985). Hepatocellular carcinoma, liver, rat. In Monographs on Pathology of Laboratory Animals. Digestive System (T. C. Jones, U. Mohr, and R. D. Hunt, eds.), pp. 39–46. Springer, New York.
Popp, J. A., and Cattley, R. C. (1991). Hepatobiliary system In Handbook of Toxicologic Pathology (W. M. Haschek and C. G. Rousseaux, eds.), pp. 279–314. Academic Press, San Diego, California.
Popper, H., Maltoni, C., and Selikoff, J. (1981). Vinyl chloride-induced hepatic lesions in man and rodents. A comparison. Liver 1, 7–20. 10.1111/j.1600-0676.1981.tb00018.x
Pozharisski, K. M., and Turusov, V. S. (1991). Angiosarcoma of the renal capsule, mouse. In Monographs on Pathology of Laboratory Animals. Cardiovascular and Musculoskeletal Systems (T. C. Jones, U. Mohr, and R. D. Hunt, eds.), pp. 91–7. Springer, New York.
Rabstein, L. S., Peters, R. L., and Spahn, G. J. (1973). Spontaneous tumors and pathologic lesions in SWR-J mice. J Natl Cancer Inst 50, 751–58. 10.1093/jnci/50.3.751
Rajtová, V., Horák, J., and Popesko, P. (2002). A Colour Atlas of the Anatomy of Small Laboratory Animals. Saunders, London.
Ramot, Y., Lewis, D., Ortel, T., Streicker, M., Moser, G., Elmore, S., Ward, S., Peddada S., and Nyska, A. (2007). Age and dose related sensitivity in 2-butoxyethanol f344 rat model of hemolytic anemia and disseminated thrombosis. Exp Toxicol Pathol 58, 311–22. 10.1016/j.etp.2006.11.003
Rank, J. M., Straka, J. G., and Bloomer, J. R. (1990). Liver in disorders of porphyrin metabolism. J Gastroenterol Hepatol 5, 573–85. 10.1111/j.1440-1746.1990.tb01443.x
Rappaport, A. M., Borowy, Z. J., Lougheed, W. M., and Lotto, W. N. (1954). Subdivision of hexagonal liver lobules into a structural and functional unit; role in hepatic physiology and pathology. Anat Rec 119, 11–33. 10.1002/ar.1091190103
Reasor, M. J., Hastings, K. L., and Ulrich, R. G. (2006). Drug-induced phospholipidosis: Issues and future directions. Expert Opin Drug Saf 5, 567–83. 10.1517/14740338.5.4.567
Reddy, J. K., Lalwani, N. D., Reddy, M. K., and Qureshi, S. A. (1982). Excessive accumulation of autofluorescent lipofuscin in the liver during hepatocarcinogenesis by methyl clofenapate and other hypolipidemic peroxisome proliferators. Cancer Res 42, 259–66. 7053853
Reed, D. J. (1998). Evaluation of chemical-inducedoxidative stress as a mechanism of hepatocyte death In Toxicology of the Liver (G. L. Plaa and W. R. Hewitt, eds.), pp. 187–220. Taylor & Francis, Washington, DC.
Rege, R. V., and Prystowsky J. B. (1998). Inflammation and a thickened mucus layer in mice with cholesterol gallstones. J Surg Res 74, 81–5. 10.1006/jsre.1997.5213
Rijhsinghani, K., Krakower, C., Swerdlow, M., Abrahams, C., and Ghose, T. (1980). Alpha-1-antitrypsin in intracellular inclusions of diethylnitrosamine induced hepatomas of C57BLxC3H F1 mice. Carcinogenesis 1, 473–79. 10.1093/carcin/1.6.473
Robison, R. L., Van Ryzin, R. J., Stoll, R. E., Jensen, R. D., and Bagdon, R. E. (1984). Chronic toxicity/carcinogenesis study of temazepam in mice and rats. Fundam Appl Toxicol 4, 394–405. 10.1016/0272-0590(84)90197-0
Rogers, A. B., and Fox, J. G. (2004). Inflammation and cancer. I. Rodent models of infectious gastrointestinal and liver cancer. Am J Physiol Gastrointest Liver Physiol 286, G361–66. 10.1152/ajpgi.00499.2003
Ruebner, B. H., Watanabe, K., and Wand, J. S. (1970). Lytic necrosis resembling peliosis hepatis produced by lasiocarpine in the mouse liver. A light and electron microscopic study. Am J Pathol 60, 247–71. PMC2032905
Ruehl-Fehlert, C., Kittel, B., Morawietz, G., Deslex, P., Keenan, C., Mahrt, C. R., Nolte, T., Robinson, M., Stuart, B. P., and Deschl, U. (2003). Revised guides for organ sampling and trimming in rats and mice—Part 1. Exp and Toxicol Pathol 55, 91–106. https://doi.org/10.1078/0940-2993-00311
Sakamoto, M., Takayama, S., and Hosoda, Y. (1989). Hemangioendothelial sarcoma in brown adipose tissue of mouse induced by carcinogenic heterocyclic amine, Glu-P-1. Toxicol Pathol 17, 754–58. 10.1177/0192623389017004203
Sasse, D., and Maly, I. P. (1991). Studies on the periportal hepatotoxicity of allyl alcohol. Prog Histochem Cytochem 23, 146–49. 10.1016/s0079-6336(11)80180-4
Sato, Y., Harada, K., Furubo, S., Kizawa, K., Sanzen, T., Yasoshima, M., Ozaki, S., Isse, K., Sasaki, M., and Nakanuma, Y. (2006). Inhibition of intrahepatic bile duct dilation of the polycystic kidney rat with a novel tyrosine kinase inhibitor gefitinib. Am J Pathol 169, 1238–250. 10.2353/ajpath.2006.051136
Sato, Y., Harada, K., Kizawa, K., Sanzen, T., Furubo, S., Yasoshima, M., Ozaki, S., Ishibashi, M., and Nakanuma, Y. (2005). Activation of the MEK5/ERK5 cascade is responsible for biliary dysgenesis in a rat model of Caroli’s disease. Am J Pathol 166, 49–60. 10.1016/S0002-9440(10)62231-6
Satoh, M., Kobayashi, K., Ishii, M., Igarashi, T., and Toyota, T. (1996). Midzonal necrosis of the liver after concanavalin A-injection. The Tohoku J Exp Med 180, 139–52. 10.1620/tjem.180.139
Schoental, R., and Magee, P. N. (1959). Further observations on the subacute and chronic liver changes in rats after a single dose of various pyrrolizidine (Senecio) alkaloids. J Pathol Bacteriol 78, 471–82. 10.1002/path.1700780213
Schulte-Hermann, R., Kraupp-Grasl, B., Bursch, W., Gerbracht, U., and Timmermann-Trosiener, I. (1989). Effects of non-genotoxic hepatocarcinogens phenobarbital and nafenopin on phenotype and growth of different populations of altered foci in rat liver. Toxicol Pathol 17, 642–50. 10.1177/0192623389017004109
Seawright, A. A. (1972). A metabolic basis for midzonal necrosis of the liver. J Pathol 107, Pxv.
Seehafer, S. S., and Pearce, D. A. (2006). You say lipofuscin, we say ceroid: Defining autofluorescent storage material. Neurobiology of Aging 27, 576–88. 10.1016/j.neurobiolaging.2005.12.006
Serra, C. A., Recalde, E. B., and Martellotto, G. I. (1987). Caroli’s disease: Congenital cystic and segmentary dilatation of the intrahepatic bile ducts. Rev Fac Cien Med Univ Nac Cordoba 45, 28–30. 3270119
Shea, S. M. (1958). The method of paired comparisons in histopathological ranking; hyaline degeneration of liver cells. A M A Arch Pathol 65, 77–80.
Shio, H., Fowler, S., Bhuvaneswaran, C., and Morris, M. D. (1982). Lysosome lipid storage disorder in NCTR-BALB/c mice. II. Morphologic and cytochemical studies. The Am J of Pathol 108, 150–59. PMC1916079
Sigal, S. H., Rajvanshi, P., Gorla, G. R., Sokhi, R. P., Saxena, R., Gebhard, D. R. Jr., Reid, L. M., and Gupta, S. (1999). Partial hepatectomy-induced polyploidy attenuates hepatocyte replication and activates cell aging events. The Am J of Physiol 276, 1260–272. 10.1152/ajpgi.1999.276.5.G1260
Singh, N. D., Sharma, A. K., Dwivedi, P., Patil, R. D., and Kumar, M. (2007). Citrinin and endosulfan induced maternal toxicity in pregnant Wistar rats: Pathomorphological study. J Appl Toxicol 27, 589–601. 10.1002/jat.1242
Sirica, A. E. (1992). The Role of Cell Types in Hepatocarcinogenesis. CRC Press, Boca Raton, Florida.
Slehria, S., Rajvanshi, P., Ito, Y., Sokhi, R. P., Bhargava, K. K., Palestro, C. J., McCuskey, R. S., and Gupta, S. (2002). Hepatic sinusoidal vasodilators improve transplanted cell engraftment and ameliorate microcirculatory perturbations in the liver. Hepatology 35, 1320–328. 10.1053/jhep.2002.33201
Solleveld, H. A., Miller, R. A., Banas, D. A., and Boorman, G. A. (1988). Primary cardiac hemangiosarcomas induced by 1,3-butadiene in B6C3F1 hybrid mice. Toxicol Pathol 16, 46–52. 10.1177/019262338801600106
Spencer, A. J., Wilson, S. A., Batchelor, J., Reid, A., Rees, J., and Harpur, E. (1997). Gadolinium chloride toxicity in the rat. Toxicol Pathol 25, 245–55. 10.1177/019262339702500301
Squire, R. A. (1989). Evaluation and grading of rat liver foci in carcinogenicity tests. Toxicol Pathol 17, 685–89. 10.1177/0192623389017004113
Squire, R. A., and Levitt, M. H. (1975). Report of a workshop on classification of specific hepatocellular lesions in rats. Cancer Res 35, 3214–223.
Srinivasan, S., Hanes, M. A., Dickens, T., Porteous, M. E., Oh, S. P., Hale, L. P., and Marchuk, D. A. (2003). A mouse model for hereditary hemorrhagic telangiectasia (HHT) type 2. Hum Mol Genet 12, 473–82. 10.1093/hmg/ddg050
Steinbrecher, U. P., Lisbona, R., Huang, S. N., and Mishkin, S. (1981). Complete regression of hepatocellular adenoma after withdrawal of oral contraceptives. Dig Dis Sci 26, 1045–050. 10.1007/BF01314771
Stejskal, R., Itabashi, M., Stanek, J., and Hruban, Z. (1975). Experimental porphyria induced by 3-(2,4,6-trimethylphenyl)-thioethyl)-4 methylsydnone. Virchows Arch B Cell Pathol 18, 83–100. 10.1007/BF02889237
Stewart, H. L. (1979). Tumours of the soft tissues. In Pathology of Tumours in Laboratory Animals. Vol. II. Tumours of the Mouse (V. S. Turusov, ed.), pp. 487–526. IARC Scientific Publications, Lyon, France.
Stewart, H. L., Williams, G., Keysser, C. H., Lombard, L. S., and Montali, R. J. (1980). Histologic typing of liver tumors of the rat. J Natl Cancer Inst 64, 177–206. https://doi.org/10.1093/jnci/64.1.177
Stinson, S.F., Hoover, K. L., and Ward, J. M. (1981). Quantitation of differences between spontaneous and induced liver tumors in mice with an automated image analyzer. Cancer Lett 14, 143–50. 10.1016/0304-3835(81)90124-5
Stout, M. D., Kissling, G. E., Suárez, F. A., Malarkey, D. E., Herbert, R. A., and Bucher, J. R. (2008). Influence of Helicobacter hepaticus infection on the chronic toxicity and carcinogenicity of triethanolamine in B6C3F1 mice. Toxicol Pathol 36, 783–94. 10.1177/0192623308322312
Tasaki, M., Umemura, T., Inoue, T., Okamura, T., Kuroiwa, Y., Ishii, Y., Maeda, M., Hirose, M., and Nishikawa, A. (2008). Induction of characteristic hepatocyte proliferative lesion with dietary exposure of Wistar Hannover rats to tocotrienol for 1 year. Toxicology 250, 143–50. 10.1016/j.tox.2008.07.003
Tepperman, J., Caldwell, F. T., and Tepperman, H. M. (1964). Induction of gallstones in mice by feeding a cholesterol-cholic acid containing diet. Am J Physiol 206, 628–34. 10.1152/ajplegacy.1964.206.3.628
Teredesai, A., Wohrmann, T., and Schlage, W. (2002). Hepatocholangiocellular carcinoma in a rat—Case report. J Vet Med A Physiol Pathol Clin Med 49, 541–44. 10.1046/j.1439-0442.2002.00497.x
Teutsch, H. F. (2005). The modular microarchitecture of human liver. Hepatology 42, 317–25. 10.1002/hep.20764
Teutsch, H. F., Schuerfeld, D., and Groezinger, E. (1999). Three-dimensional reconstruction of parenchymal units in the liver of the rat. Hepatology 29, 494–505. 10.1002/hep.510290243
Tian, X. F., Fan, X. G., Fu, C. Y., Huang, Y., and Zhu, C. (2005). Experimental study on the pathological effect of Helicobacter pylori on liver tissues. Zhonghua Gan Zang Bing Za Zhi 13, 780–83.
Tillmann, T., Kamino, K., Dasenbrock, C., Ernst, H., Kohler, M., Morawietz, G., Campo, E., Cardesa, A., Tomatis, L., and Mohr, U. (1997). Subcutaneous soft tissue tumours at the site of implanted microchips in mice. Exp Toxic Pathol 49, 197–200. 10.1016/S0940-2993(97)80007-3
Travlos, G. S., Mahler, J., Ragan, H. A., Chou, B. J., and Bucher, J. R. (1996). Thirteen-week inhalation toxicity of 2- and 4-chloronitrobenzene in F344/N rats and B6C3F1 mice. Fund and Appl Toxicol 30, 75–92. 10.1006/faat.1996.0045
Trotman, B. W., Bernstein, S. E., Balistreri, W. F., and Martin, R. A. (1983). Interaction of hemolytic anemia and genotype on hemolysis-induced gallstone formation in mice. Gastroenterology 84,719–24. https://doi.org/10.1016/0016-5085(83)90136-1
Tschudy, D. P. (1962). Biochemical studies of experimental porphyria. Metabolism 11, 1287–301.
Tsokos, M., and Erbersdobler, A. (2005). Pathology of peliosis. Forensic Sci Int 149, 25–33. https://doi.org/10.1016/j.forsciint.2004.05.010
Turusov, V. S., Day, N. E., Tomatis, L., Gati, E., and Charles, R. T. (1973). Tumors in CF-1 mice exposed for six consecutive generations to DDT. J Natl Cancer Inst 51, 983–97. 10.1093/jnci/51.3.983
Turusov, V. S., Deringer M. K., Dunn T. B., and Stewart H. L. (1973). Malignant mouse-liver tumors resembling human hepatoblastomas. J Natl Cancer Inst 51, 1689–695. 10.1093/jnci/51.5.1689
Ungar, H. (1986). Venoocclusive disease of the liver and phlebectatic peliosis in the golden hamster exposed to dimethylnitrosamine. Pathol Res Pract 181, 180–87. 10.1016/S0344-0338(86)80008-5
Ungar, H., Sullman, S. F., and Zuckerman A. J. (1976). Acute and protracted changes in the liver of Syrian hamsters induced by a single dose of aflatoxin B1. Observations on pathological effects of the solvent (dimethylformamide). Brit J of Exp Pathol 57, 157–64. PMC2041104
van Zwieten, M. J., and Hollander, C. F. (1997). Intranuclear and intracytoplasmic inclusions, liver, rat. In Monographs on Pathology of Laboratory Animals: Digestive System (T. C. Jones, J. A. Popp, and U. Mohr eds.), pp. 133–39. Springer, New York.
Vesselinovitch, S. D., Mihailovich, N., and Rao, K. V. N. (1978). Morphology and metastatic nature of induced hepatic nodular lesions in C57BL x C3H F1 mice. Cancer Res 38, 2003–010. http://cancerres.aacrjournals.org/cgi/content/abstract/38/7/2003
Vollmar, B., Siegmund, S., Richter, S., and Menger, M. D. (1999). Microvascular consequences of Kupffer cell modulation in rat liver fibrogenesis. J Pathol 189, 85–91. 10.1002/(SICI)1096-9896(199909)189:1<85::AID-PATH399>3.0.CO;2-1
Vons, C., Beaudoin, S., Helmy, N., Dagher, I., Weber, A., and Franco, D. (2009). First description of the surgical anatomy of the cynomolgus monkey liver. Am J of Primatol 71, 400–08. 10.1002/ajp.20667
Vowles, G. H., and Francis, R. J. (2002). Amyloid. In Theory and Practice of Histological Techniques (J. D. Bancroft and M. Gamble, eds.), pp. 303–24. Churchill Livingstone, Edinburgh, Scotland.
Walker, R. M., Racz, W. J., and McElligott, T. F. (1985). Acetaminophen-induced hepatotoxic congestion in mice. Hepatology 5, 233–40. 10.1002/hep.1840050213
Wang, J., Zhou, G., Chen, C., Yu, H., Wang, T., Ma, Y., Jia, G., Gao, Y., Li, B., Sun, J., Li, Y., Jiao, F., Zhao, Y., and Chai, Z. (2007). Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett 168, 176–85. 10.1016/j.toxlet.2006.12.001
Ward, J. M. (1981). Morphology of foci of altered hepatocytes and naturally occurring hepatocellular tumors in F344 rats. Virchows Arch A 390, 339–45. 10.1007/BF00496563
Ward, J. M. (1997). Cirrhosis, mouse. In Monographs on Pathology of Laboratory Animals: Digestive System (T. C. Jones, J. A. Popp, and U. Mohr, eds.), pp. 151–54. 2nd Edition. Springer, New York.
Ward, J. M., Anver, M. R., Haines, D. C., and Benveniste, R. E. (1994). Chronic active hepatitis in mice caused by Helicobacter hepaticus. Am J Pathol 145, 959–68. PMC1887338
Ward, J. M., Collins, M. J., Jr., and Parker, J. C. (1977). Naturally occuring mouse hepatitis virus infection in nude mouse. Lab Anim Sci 27, 372–76.
Ward, J. M., Diwan, B. A., Ohshima, M., Hu, H., Schuller, H. M., and Rice, J. M. (1986). Tumor-initiating and promoting activities of di(2-ethylhexyl) phthalate in vivo and in vitro. Environ Health Perspect 65, 279–91. 10.1289/ehp.8665279
Ward, J. M., Fox, J. G., Anver, M. R., Haines, D. C., George, C. V., Collins, M. J., Jr., Gorelick, P. L., Nagashima, K., Gonda, M. A., Gilden, R. V. et al. (1994b). Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J Natl Cancer Inst 86, 1222–227. 10.1093/jnci/86.16.1222
Ward, J. M., Goodman, D. G., Squire, R. A., Chu, K. C., and Linhart, M. S. (1979). Neoplastic and nonneoplastic lesions in aging (C57BL/6N x C3H/HeN)F1 (B6C3F1) mice. J Natl Cancer Inst 63, 849–54. 10.1093/jnci/63.3.849
Ward, J. M., Lynch, P., and Riggs, C. (1988). Rapid development of hepatocellular neoplasms in aging male C3H/HeNCr mice given phenobarbital. Cancer Lett 39, 9–18. 10.1016/0304-3835(88)90035-3
Ward, J. M., Rice, J. M., Creasia, D., Lynch, P., and Riggs, C. (1983). Dissimilar patterns of promotion by di(2-ethylhexyl)phthalate and phenobarbital of hepatocellular neoplasia initiated by diethylnitrosamine in B6C3F1 mice. Carcinogenesis 4, 1021–029. 10.1093/carcin/4.8.1021
Ward, J. M., Wobus, C. E., Thackray, L. B., Erexson, C. R., Faucette, L. J., Belliot, G., Barron, E. L., Sosnovtsev S. V., and Green K. Y. (2006). Pathology of immunodeficient mice with naturally-occurring murine norovirus infection. Toxicol Path 34, 708–15. 10.1080/01926230600918876
Ward, J. M., Yoon, M., Anver, M. R., Haines, D. C., Kudo, G., Gonzalez, F. J., and Kimura, S. (2001). Hyalinosis and Ym1/Ym2 gene expression in the stomach and respiratory tract of 129S4/SvJae and wild-type and CYP1A2-null B6, 129 mice. Am J Pathol 158, 323–32. 10.1016/S0002-9440(10)63972-7
Wilson, D. M., Goldsworthy, T. L., Popp, J. A., and Butterworth, B. E. (1992). Evaluation of genotoxicity, pathological lesions, and cell proliferation in livers of rats and mice treated with furan. Environ Mol Mutagen 19, 209–22. 10.1002/em.2850190305
Wilson, D., Lame, M., Dunston, S., and Segall, H. (2000). DNA damage cell checkpoint activities are altered in monocrotaline pyrrole-induced cell cycle arrest in human pulmonary artery endothelial cells. Toxicol Appl Pharmacol 166, 69–80. 10.1006/taap.2000.8966
Wolstenholme, J. T., and Gardner, W. U. (1950). Sinusoidal dilatation occurring in livers of mice with a transplanted testicular tumor. Proc Soc Exp Biol Med 74, 659–61. 10.3181/00379727-74-18007
Wong, A. K., Alfert, M., Castrillon, D. H., Shen, Q., Holash, J., Yancopoulos, G. D., and Chin, L. (2001). Excessive tumor-elaborated VEGF and its neutralization define a lethal paraneoplastic syndrome. Proc Natl Acad Sci U.S.A 98, 7481–486. 10.1073/pnas.121192298
World Health Organization. (1978). Principles and Methods for Evaluating the Toxicity of Chemicals. Part I. World Health Organization, Geneva.
Wright, P. F., and Stacey, N. H. (1991). A species/strain comparison of hepatic natural lymphocytotoxic activities in rats and mice. Carcinogenesis 12, 1365–370. 10.1093/carcin/12.8.1365
Xie, Y., Newberry, E. P., Kennedy, S. M., Luo, J., and Davidson, N. O. (2009). Increased susceptibility to diet-induced gallstones in liver fatty acid binding protein (L-Fabp) knockout mice. J Lipid Res 50, 977–87. 10.1194/jlr.M800645-JLR200
Xu, C., Li, C. Y., and Kong, A. N. (2005). Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res 28, 249–68. 10.1007/BF02977789
Xu, S. X., Zheng, D., Sun, Y. M., Wang, S. H., Shao, L. Q., Huang, Q. Y., and Xiang, D. L. (1992). Subchronic toxicity studies of fenbendazole in rats. Vet Hum Toxicol 34, 411–13.
Yamate, J., Tajima, M., Ihara, M., Shibuya, K., and Kudow, S. (1988). Spontaneous vascular endothelial cell tumors in aged B6C3F1 mice. Jpn J Vet Sci 50, 453–61. 10.1292/jvms1939.50.453
Yang, Y. H., and Campbell, J. S. (1964). Crystalline excrements in bronchitis and cholecystitis of mice. Am J Pathol 45, 337–45. PMC1907179
Yasui, M., Yase, Y., and Ota, K. (1991). Distribution of calcium in central nervous system tissues and bones of rats maintained on calcium-deficient diets. J Neurol Sci 105, 206–10. 10.1016/0022-510x(91)90146-x
Yoshitomi, K., Alison, R. H., and Boorman, G. A. (1986). Adenoma and adenocarcinoma of the gallbladder in aged laboratory mice. Vet Pathol 23, 523–27. 10.1177/030098588602300428
Yoshitomi, K., and Boorman, G. A. (1994). Tumours of the gallbladder. In Pathology of Tumours in Laboratory Animals. Vol. 2. Tumours of the Mouse (V. Turusov and U. Mohr, eds.), pp. 271–79, 2nd edition. IARC Scientific Publications, Lyon, France.
Yu, W., Wan, X., Wright, J. R., Jr., Coddington, D., and Bitter-Suermann, H. (1994). Heterotopic liver transplantation in rats: Effect of intrahepatic islet isografts and split portal blood flow on liver integrity after auxiliary liver isotransplantation. Surgery 115, 108–17.
Zatloukal, K., Stumptner, C., Lehner, M., Denk, H., Baribault, H., Eshkind, L. G., and Franke, W. W. (2000). Cytokeratin 8 protects from hepatotoxicity, and its ratio to cytokeratin 18 determines the ability of hepatocytes to form Mallory bodies. Am J Pathol. 156, 1263–274. 10.1016/S0002-9440(10)64997-8
Zenner, L. (1999). Pathology, diagnosis and epidemiology of the rodent Helicobacter infection. Comp Immunol Microbiol Infect Dis 22, 41–61. 10.1016/s0147-9571(98)00018-6
Zimmerman, H. J. (1998). Drug-induced hepatic disease. In Toxicology of the Liver (G. L. Plaa, and W. R. Hewitt, eds.), pp. 3–60. Taylor & Francis, Washington, DC.
Zwicker, G. M., Eyster, R. C., Sells, D. M., and Gass, J. H. (1995). Spontaneous vascular neoplasms in aged Sprague-Dawley rats. Toxicol Pathol 23, 518–26. 10.1177/019262339502300409