Global Pathology Support B.V.
毒理病理学
愿景声明
药物、化学品和疫苗都是安全的,没有或至少可能没有负面影响。
任务说明
在 GPS B.V.,我们通过开展毒理病理学研究来确定药物、化学品和疫苗的最安全剂量。
自豪的会员
NVVP – 荷兰病理学会
NVT – 荷兰毒理学会
STP – 毒理学病理学会
SOT – 毒理学会
ESTP – 欧洲毒理学病理学会

- 30+
工作年限
- 2000+
服务对象
Global Pathology Support B.V.
Global Pathology Support (GPS) 是一家咨询公司,于 2004 年在荷兰成立。 GPS 专门从事毒理学研究中化学品的临床前病理学评估,以进行安全评估。
我们的服务重点是(生物)制药和化工行业为药品和疫苗等注册而开发的产品的安全性。 全球定位系统还根据化学品注册、评估和授权(REACH)协议提供服务,按照经合组织准则对化学品进行安全评估。
全球定位系统一直致力于帮助我们的客户实现其开发目标,除了使用光学显微镜进行传统组织病理学检查外,我们现在还可以在全球范围内提供创新的 GLP 验证数字病理学检查、全面组织病理学评估和临床前毒理学研究同行评审解决方案。 该选项(数字病理学)现在也可通过大量整张切片图像格式(如 Hamamatsu、3DHistech、Olympus、Aperio/Leica、Ventana/Roche、Philips、Zeiss 扫描仪等)的组织学切片扫描结果执行。
我们的服务
我们采用最高质量标准,使用最好的病理系统之一(Provantis® - Instem LSS)进行组织病理学评估,以灵活、快速和高效的方式为您提供服务。
数字病理学
也称为远程病理学:由毒物病理学家根据组织玻璃切片的整片图像(W.S.I.)进行组织病理学评估。
毒理病理学咨询
Global Pathology Support 通过了经合组织良好实验室规范(包括数字病理学)原则的 GLP 合规认可认证。 组织病理学评估由委员会认证的毒理学病理学家进行。
生物制药咨询
Global Pathology Support 拥有开展生物制药咨询所需的全部经验和声望。
大体病理评估
Global Pathology Support 是荷兰为数不多的能够正确执行大体病理评估的合同实验室之一。
神经病理学评估
全球病理学支持部门在评估临床前毒性研究(如 OECD 443)的神经病理学方面拥有丰富的专业知识和经验。
病理学同行评审
多年来,我们的服务已扩展到组织病理学审查、病理学同行评审(GLP)、毒理病理学和神经病理学评估方面的建议和咨询。
您好,我是 Bob Thoolen 博士,一位经验丰富的委员会认证毒理学病理学家,拥有超过 25 年的专业知识。 在 Global Pathology Support B.V. (GPS),我们走在确保药物、疫苗和化学品临床前安全性的最前沿。 我们致力于提供高质量、具有成本竞争力的毒理病理学服务,并严格遵守经合组织良好实验室规范等国际准则。
请联系我们位于荷兰海牙的总部,或 联系我们探索我们病理学服务的深度。 加入 Global Pathology Support B.V.,与我们一起打造临床前安全的未来。
Dr. R.J.M.M. Thoolen
Board Certified Toxicologic Pathologist (PhD, CRP/TP) / Test Facility Manager
在此声明,我认识 Thoolen 博士已有数年之久,既是他的同事,也是他的朋友。 他在荷兰索尔维制药公司(Solvay Pharmaceutical, BV)担任首席病理学家期间,以及后来在荷兰全球病理支持公司(Global Pathology Support)工作期间,我曾与他共事。 罗伯特是一位知名的毒理学病理学家,在常规病理工作和同行评审报告方面经验丰富。 他的敬业精神和工作质量都达到了很高的标准。 罗伯特待人友好,平易近人,是一个很容易相处的人。
他在本行业内工作出色,他对 STP 命名小组在肝脏这一重要主题上所做的贡献深受赞赏。
我祝愿他在毒物病理学领域继续创业成功。
我和我的同事们一直都很感谢与鲍勃的合作,他是我们的外部病理咨询专家之一,他提供了可靠的、完全符合 GLP 标准的组织病理学支持,并及时灵活地遵守最后期限。
我们多次与 Thoolen 博士合作。 他总是能在很短的时间内提供准确的幻灯片评估。 他彬彬有礼,知识渊博,即使在具有挑战性的项目中,我们也很容易与他合作。
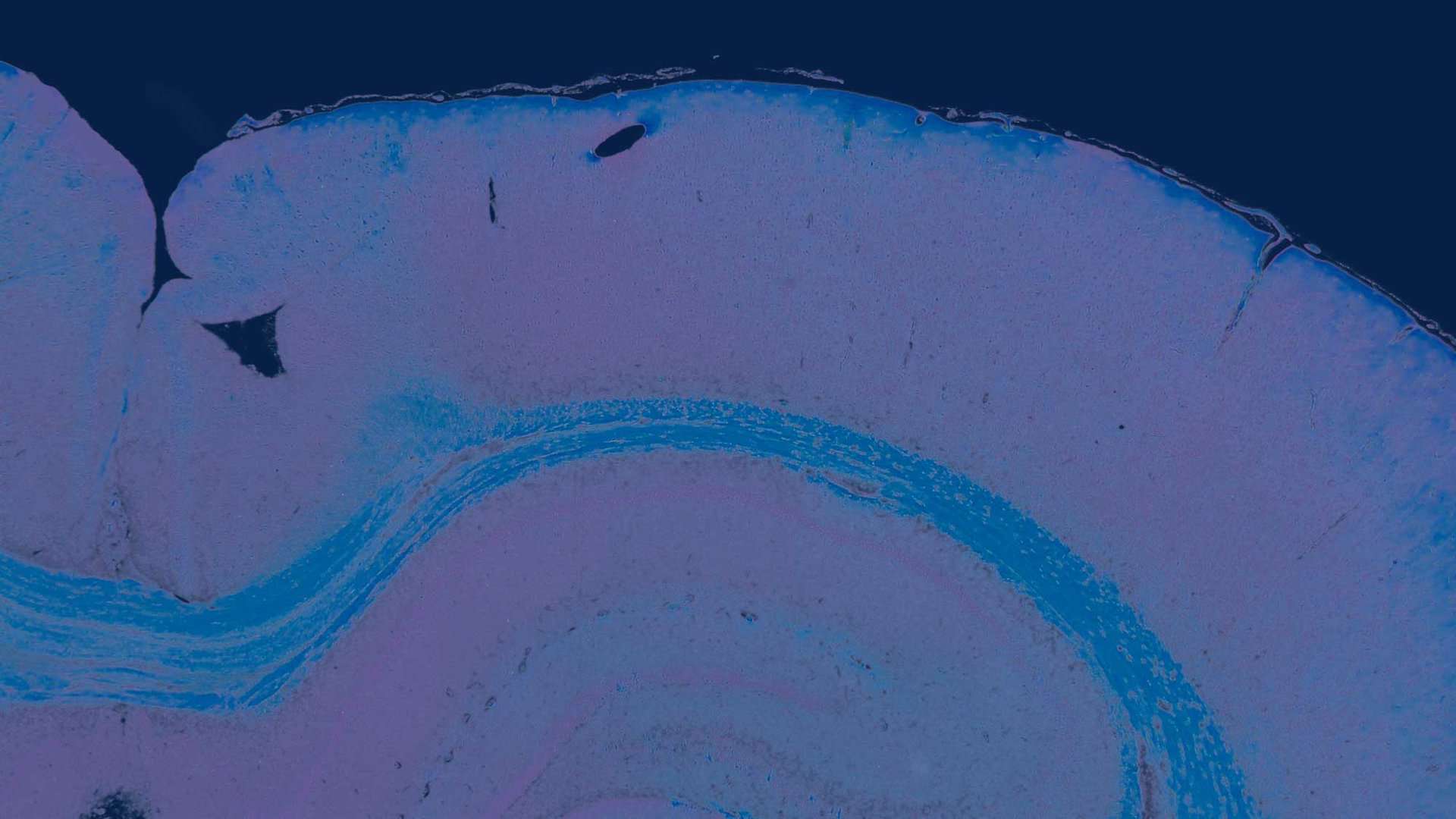
毒理病理学家在全球病理支持机构安全性评估中的关键作用
从尸体解剖到组织病理学评估、数字病理学的解释和使用
毒理病理学–它的含义和病理学家的作用是什么?
在使用制药业的医药产品方面,用户的体验应该是积极的,负面副作用应尽可能减少。 为了评估负面影响,需要进行毒理学研究。 对物质毒性的研究旨在通过设定限值来预防疾病,低于限值的健康风险可忽略不计或为零。 毒理学)病理学家在评估疫苗、药品和化学品的安全性方面发挥着至关重要的作用。 重点是病理变化的鉴定、这些变化的评估和相关性,特别是在将这些动物实验数据应用于人类(风险评估)的背景下对这些数据的解释。 上个世纪发生的灾难,例如在子宫内使用沙利度胺(致畸作用)和合成雌激素己烯雌酚(DES)(致癌物)后发生的灾难,告诉我们警惕药物的副作用是多么重要。 此外,还必须监测杀虫剂和工业废物对食物来源的污染。 某些杀虫剂(滴滴涕和狄氏剂)等化合物已被证明在实验室动物体内致癌(通常剂量相对较高),但在人类日常食物中的含量较低。 此外,受工业污染的物质(如多氯联苯、全氟和多氟烷基物质 (PFAS))也需要进行全面的临床前安全评估。 此外,越来越多的药物在上市后退出流通,这也凸显了安全性评估的重要性,而毒理病理学家在其中发挥着至关重要的作用。
在 Global Pathology Support B.V.,我们以符合 GLP(良好实验室规范)的方式提供临床前病理学支持。 用于毒理学研究的组织病理学评估,对所测试化合物的安全性评估起着重要作用。 我们还使用 “全切片图像”(W.S.I.),由获得认证的毒理病理学家对非临床 GLP 研究进行评估。
全切片图像(W.S.I.)已成为毒理病理学评估非临床 GLP 研究的重要现代工具。 在毒理病理学中使用 W.S.I. 彻底改变了病理数据的收集、分析和交流方式。 由毒理病理学家对幻灯片(W.S.I.)扫描进行评估的额外选择不仅效率更高,而且还有其他好处。
良好实验室规范(GLP)
全球病理学支持(GPS)在临床前毒性和致癌性研究中提供科学、准确和符合 GLP 的病理学数据方面发挥着至关重要的作用。 为此,法律规定必须建立符合 GLP 标准的质量体系。 全球定位系统按照 “良好实验室规范”(Good Laboratory Practice)运行至今已有 19 年之久。
这就保证了我们的客户能够从全球超过 38 个国家的全球定位系统中获得良好的病理报告。 这些报告可随时用于根据相关国家当局的规定进行登记。










